Ethics in Research презентация
Содержание
- 2. Ethics Ethics – the discipline concerned with what is morally good
- 3. Definition of Scientific Misconduct Scientific misconduct is fabrication, falsification, or plagiarism
- 4. Codes and Guidelines 1974 – US Congress formed the National Commission
- 5. Codes and Guidelines, cont’d Codes and guidelines evolved because of human
- 6. Institutional Review Board (IRB) IRB is a panel of research experts
- 7. Statement of Informed Consent Provides potential subject with information to make
- 8. Statement of Informed Consent, cont’d Elements of Informed Consent Document: Background
- 9. Statement of Informed Consent, cont’d Other components sometimes included in the
- 10. Seven Areas of Scientific Dishonesty Plagiarism Fabrication and falsification Nonpublication of
- 11. Plagiarism Plagiarism—using the ideas, writings, and drawings of others as your
- 12. Fabrication and Falsification Fabrication and falsification—making up or altering data Prominent
- 13. Researcher Faces Prison for Fraud in NIH Grant Applications and Papers
- 14. Nonpublication of Data Sometimes called “cooking data” Data not included in
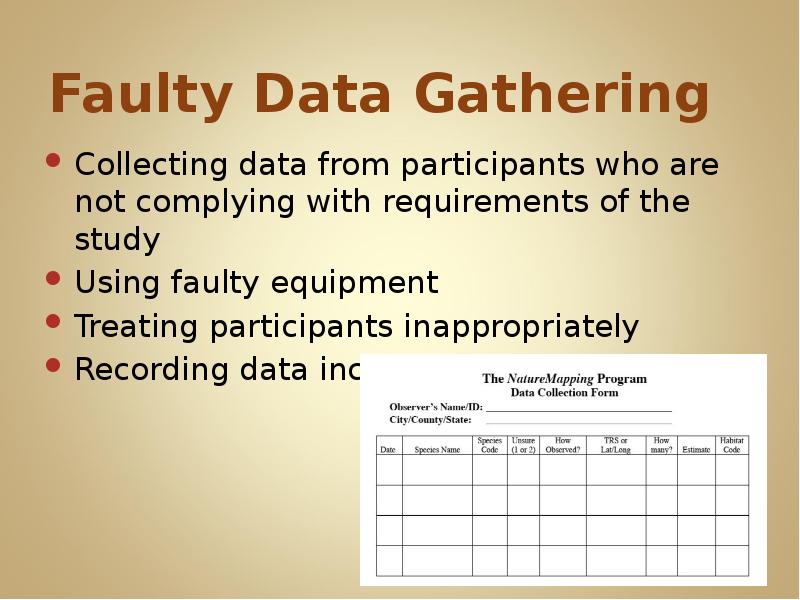
- 15. Faulty Data Gathering Collecting data from participants who are not complying
- 16. Data Gathering Most important and most aggravating. Always drop non-compliers. Fix
- 17. Poor Data Storage and Retention Data should be stored in its
- 18. Misleading Authorship Misleading authorship—who should be an author? Technicians do not
- 19. MSSE Information for Authors Medicine & Science in Sports & Exercise®
- 20. Sneaky Publication Practices Publication of the thesis or dissertation Should be
- 21. Sanctions Freeze your job. Reduce your job. Lose your job. Loss
- 22. Why do it? Obtain external funding. Pressure of the job. Need
- 23. Working With Faculty Expect to be treated with respect but not
- 24. Case Example - Pat J. Palmer Fabricated 6 interview records Fabricated
- 25. Next Class Abstract due Chapter 6-basic stats
- 26. Скачать презентацию























Слайды и текст этой презентации
Похожие презентации





























