Pharmacovigilance. Marta D. Puente Navazo January 2017 презентация
Содержание
- 2. Agenda Pharmacovigilance Definitions Reporting details Local literature surveillance
- 3. Pharmacovigilance Pharmacovigilance Definitions Reporting details Local literature surveillance
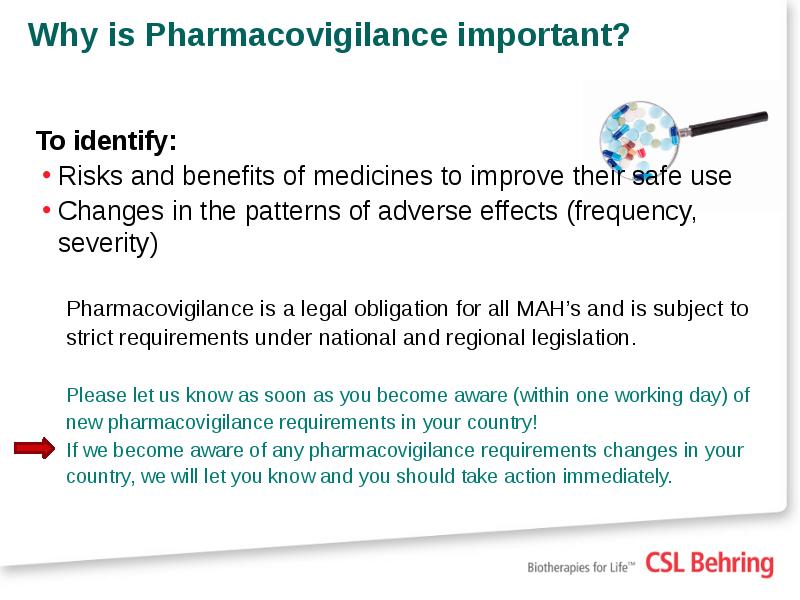
- 4. Why is Pharmacovigilance important? To identify: Risks and benefits of medicines
- 5. Why is Pharmacovigilance important? CSL has a regulatory
- 6. Definitions Pharmacovigilance Definitions Reporting details Local literature surveillance
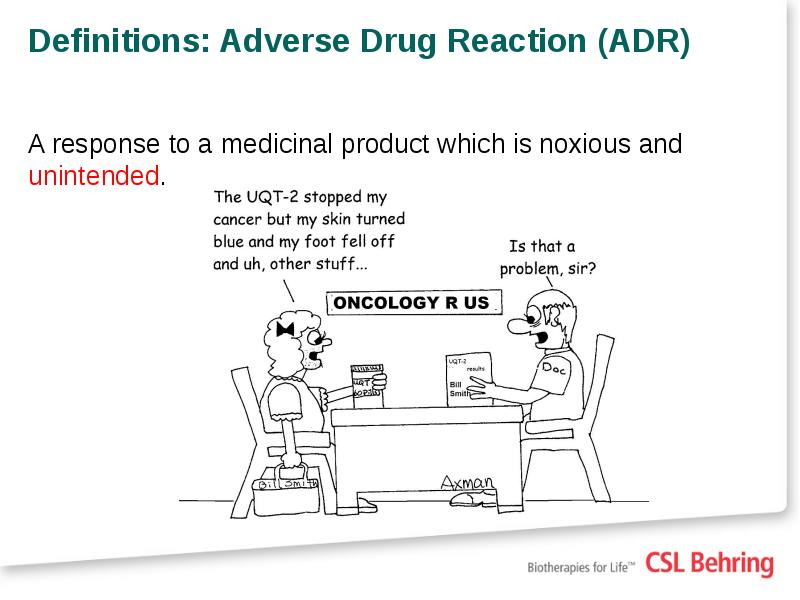
- 7. Definitions: Adverse Drug Reaction (ADR) A response to a medicinal
- 8. Definitions- Adverse Event or Adverse Experience (AE) Is any untoward
- 9. Definitions – Adverse Event Report In addition to Adverse events,
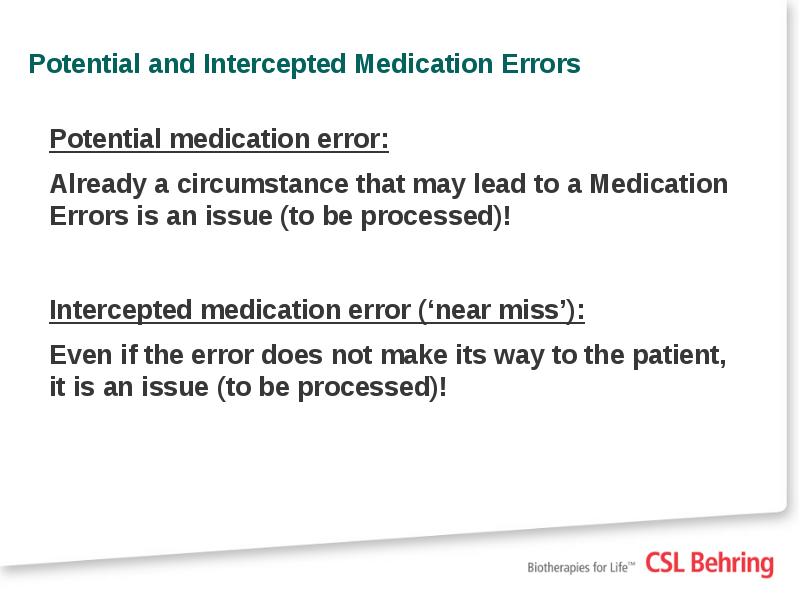
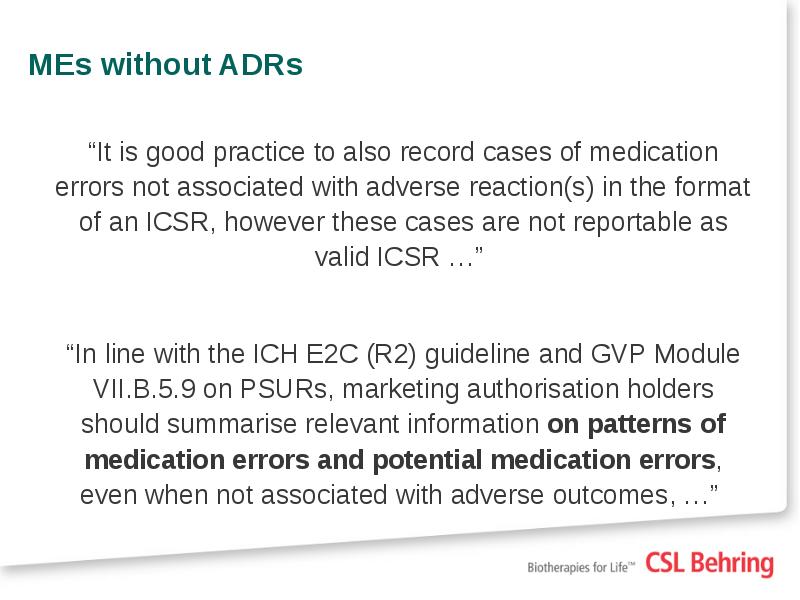
- 10. Additional guidance on medication errors
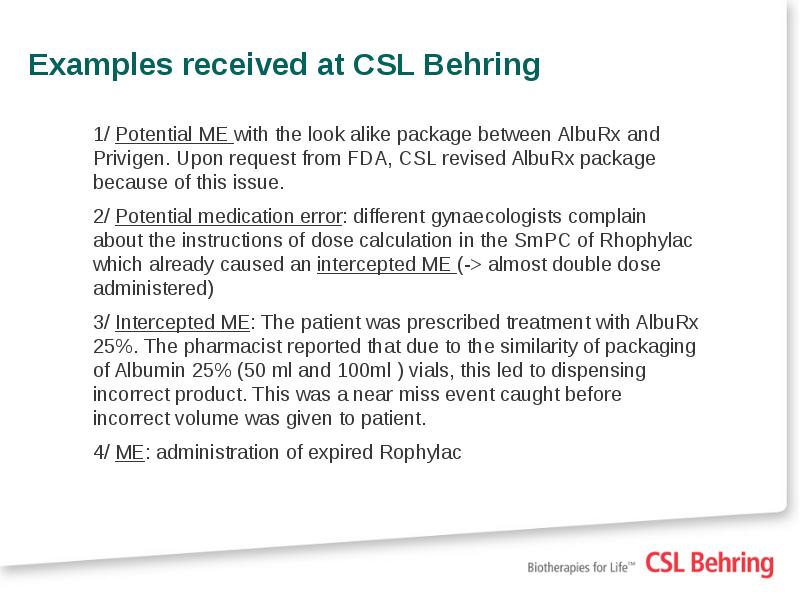
- 14. EXAMPLES
- 15. Need to be reported? Midwife administered a dose of Rhophylac
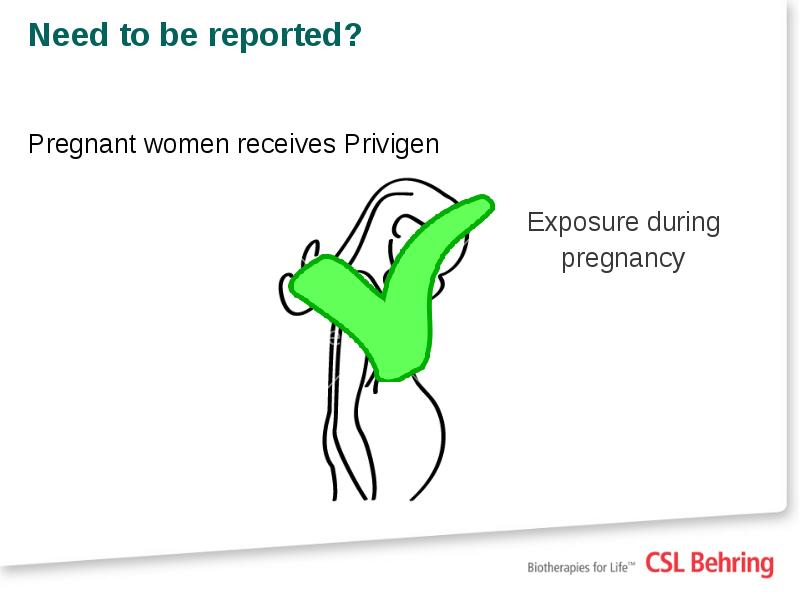
- 16. Need to be reported? Pregnant women receives Privigen
- 17. Need to be reported? The patient had a poor response to
- 18. Need to be reported? Treatment with Kybernin of preeclampsia in pregnant
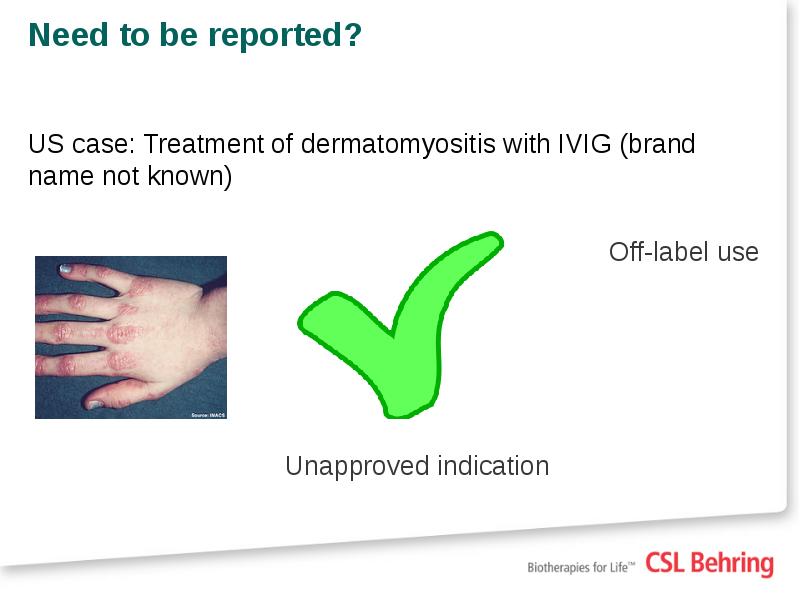
- 19. Need to be reported? US case: Treatment of dermatomyositis with IVIG
- 20. Need to be reported? Breastfeeding baby was exposed to Privigen
- 21. Need to be reported? Haemate was transferred into the syringe and
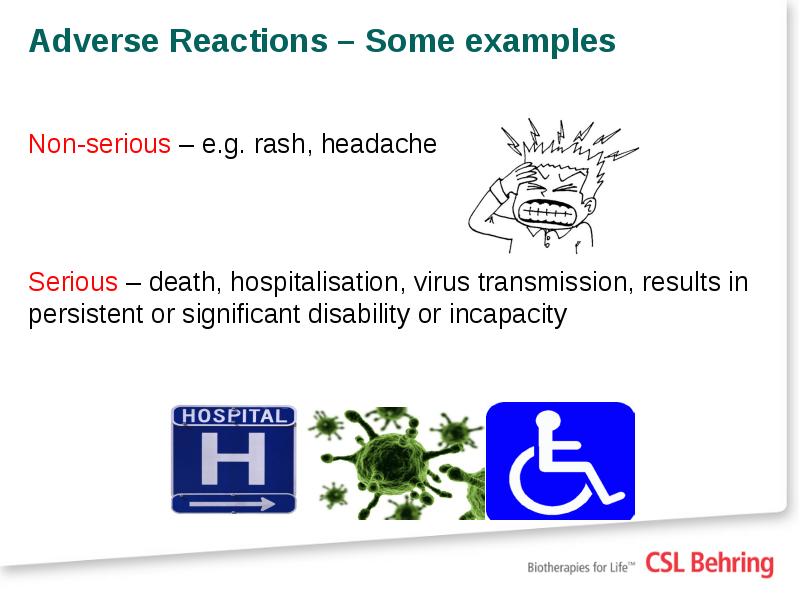
- 22. Adverse Reactions – Some examples Non-serious – e.g. rash, headache Serious
- 23. Definitions
- 24. Individual Case Safety Report (ICSR)
- 25. Spontaneous ICSR
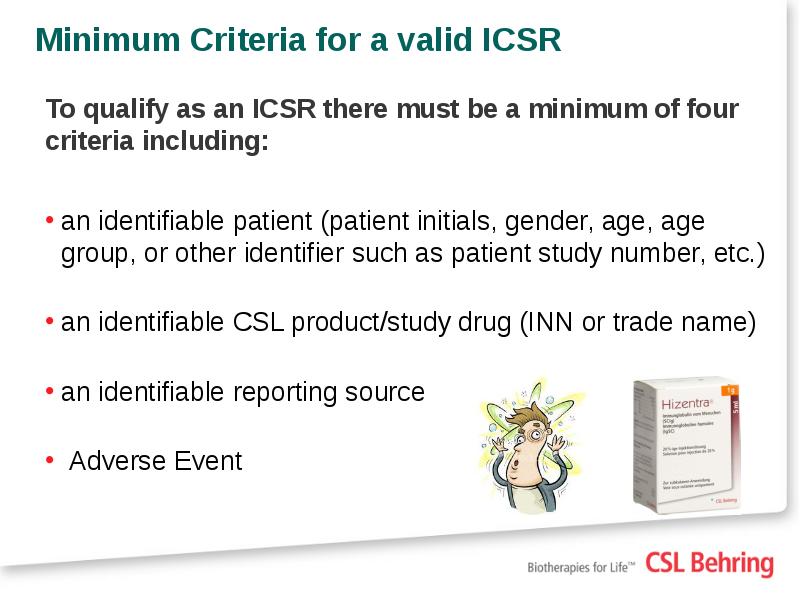
- 26. Minimum Criteria for a valid ICSR
- 27. ICSR reporting
- 28. Reporting details Pharmacovigilance Definitions Reporting details Local literature surveillance
- 29. Pharmacovigilance – LSO/RSO Contacts Regional Safety Officer (RSO) ECI: Marta
- 30. An Adverse Drug Reaction (ADR) – What Should I Do? Post
- 31. Adverse Reactions – What Should I Do? If phone calls
- 32. Important to know: Every information on a potential AE
- 33. Adverse Reactions – Reporting Timelines All adverse reactions (ADR) must
- 34. Product Exposure during Pregnancy Pregnancy reports should be monitored until the
- 35. An example
- 36. If you suspect an ADR… If you suspect an ADR…
- 37. Conference:
- 38. “European Journal of Neurology” Attending a conference you come across an
- 39. Local Literature surveillance Pharmacovigilance Definitions Reporting details Local literature
- 40. Why local literature surveillance? Information on safety relevant observations in
- 41. Local literature surveillance 3. If you find an abstract mentioning a
- 42. Local literature surveillance Example Rhophylac: Global literature search uses following
- 44. Скачать презентацию
































Слайды и текст этой презентации
Скачать презентацию на тему Pharmacovigilance. Marta D. Puente Navazo January 2017 можно ниже:
Похожие презентации