The second law of thermodynamics презентация
Содержание
- 2. Irreversibility of processes There exist many processes that are irreversible: the
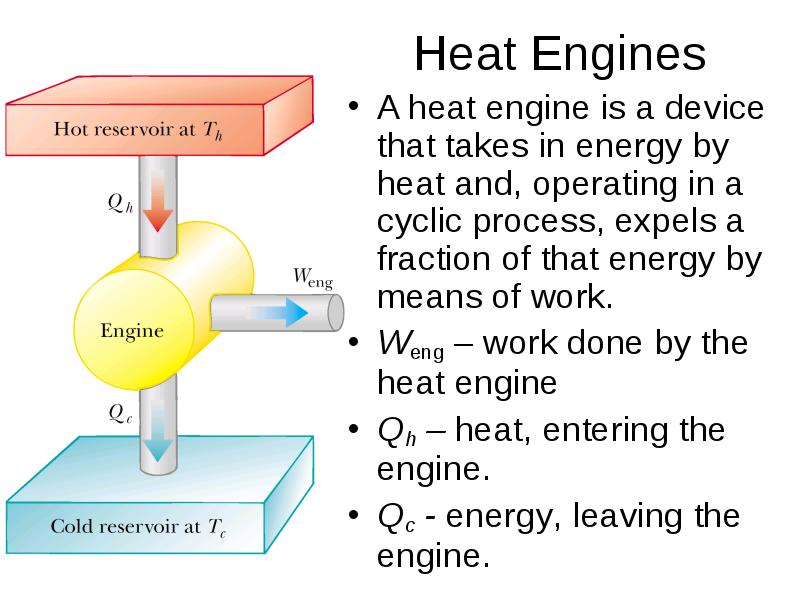
- 3. Heat Engines A heat engine is a device that takes in
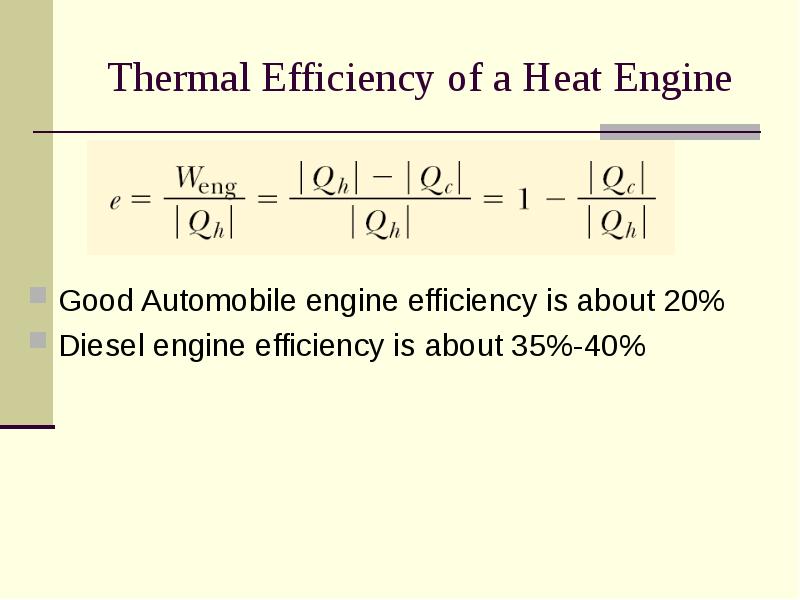
- 4. Thermal Efficiency of a Heat Engine Good Automobile engine efficiency is
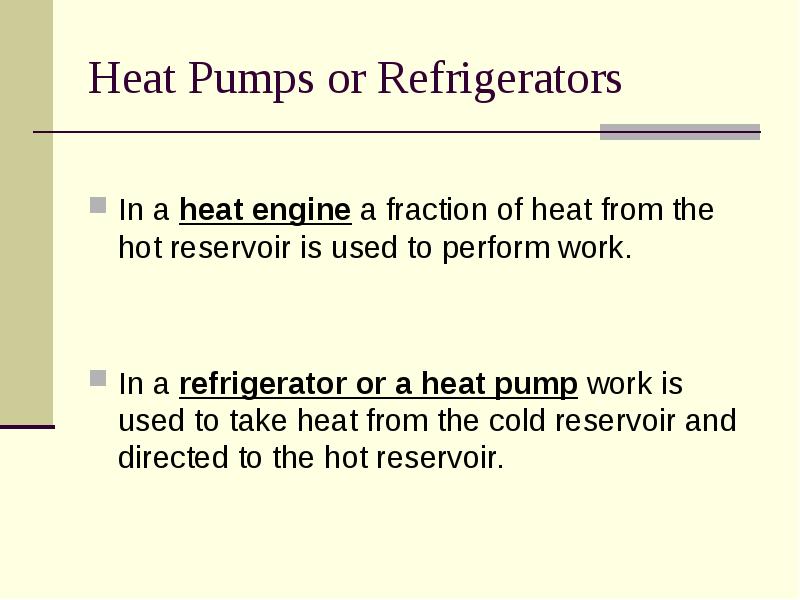
- 5. Heat Pumps or Refrigerators In a heat engine a fraction of
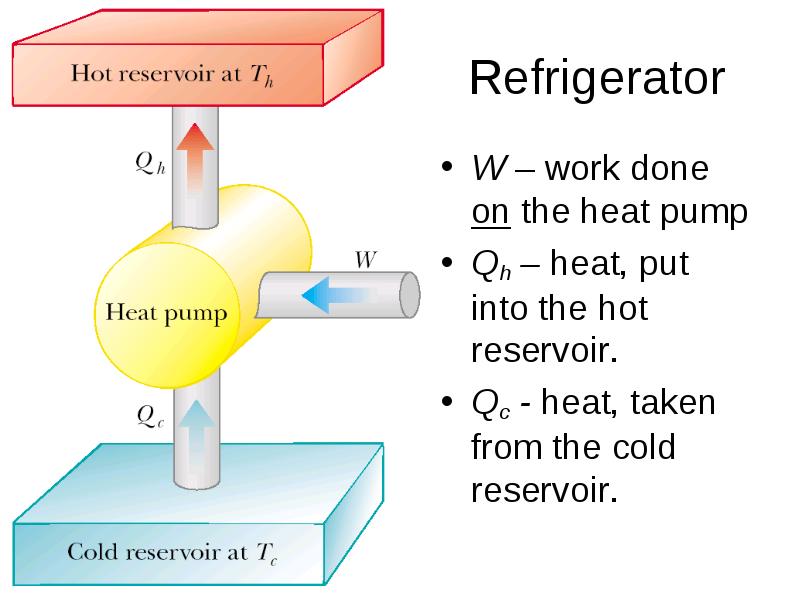
- 6. Refrigerator W – work done on the heat pump Qh
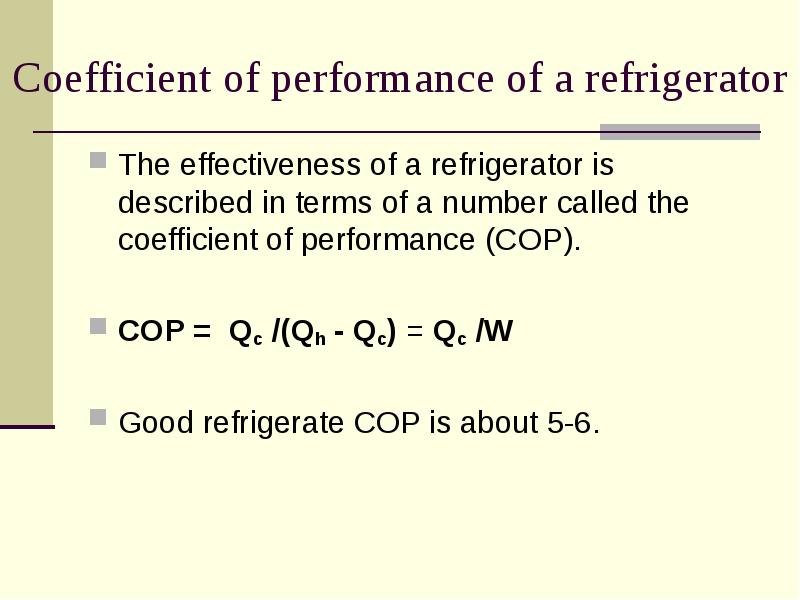
- 7. Coefficient of performance of a refrigerator The effectiveness of a refrigerator
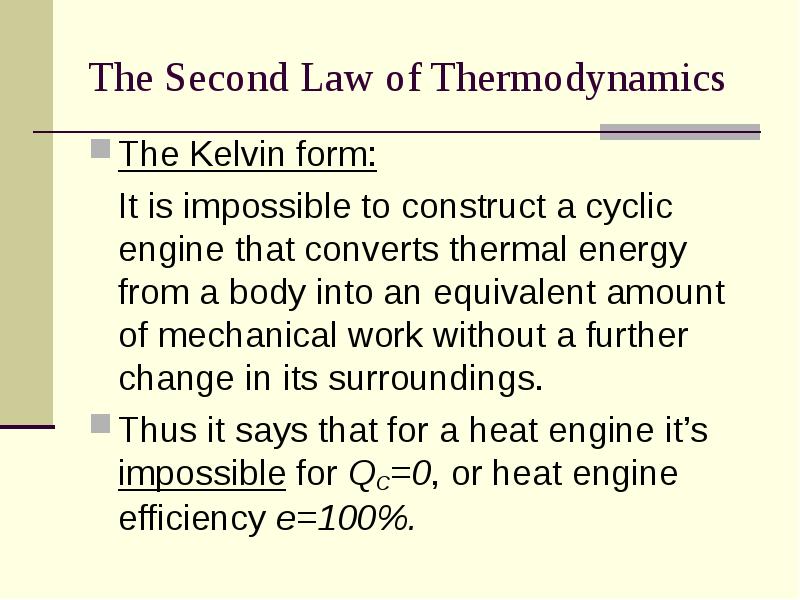
- 8. The Second Law of Thermodynamics The Kelvin form: It is
- 9. The Second Law of Thermodynamics The Clausius form: It is impossible
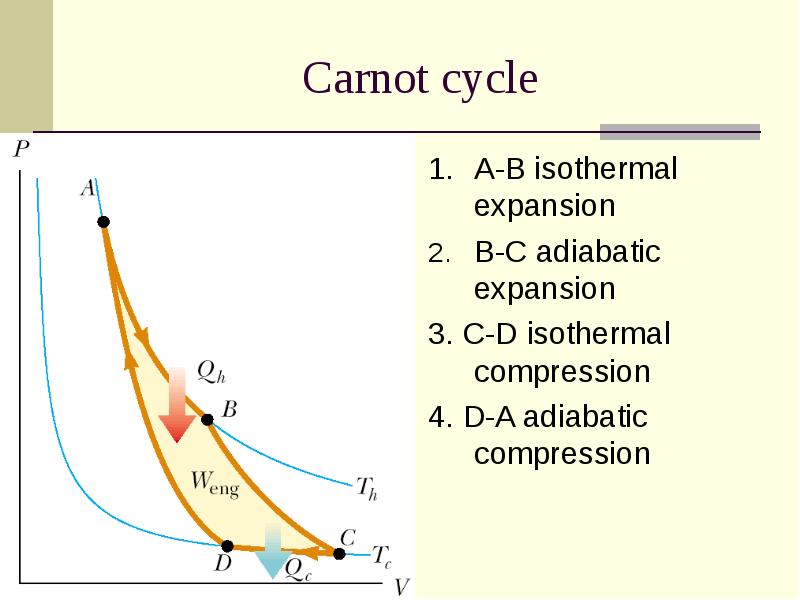
- 10. Carnot cycle 1. A-B isothermal expansion B-C adiabatic expansion 3. C-D isothermal
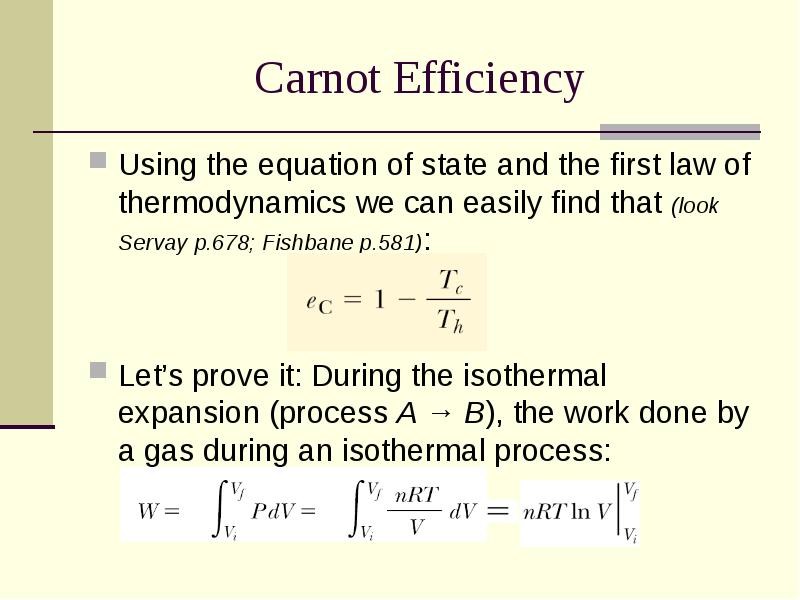
- 11. Carnot Efficiency Using the equation of state and the first law
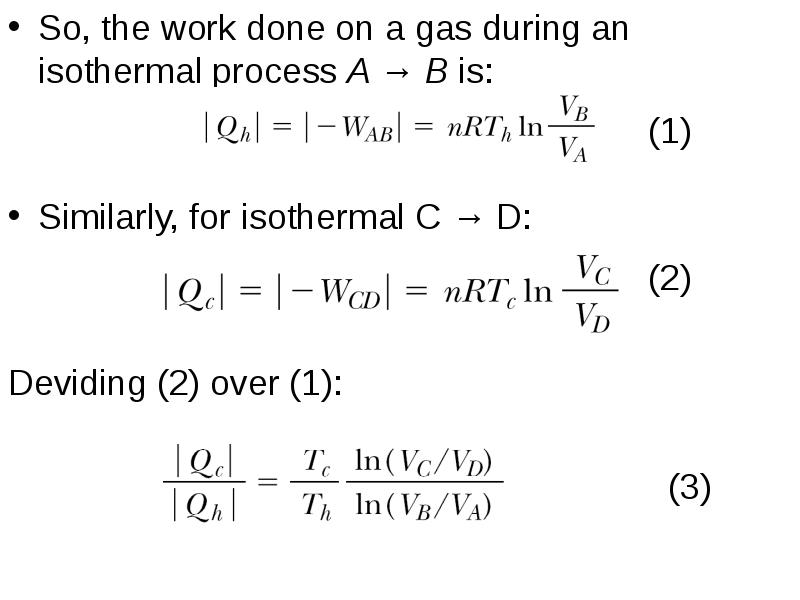
- 12. So, the work done on a gas during an isothermal process
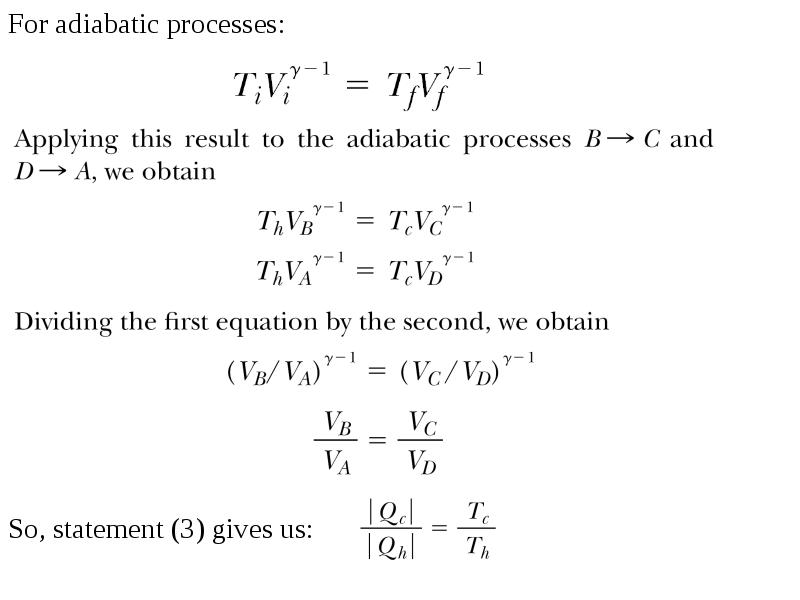
- 13. For adiabatic processes: For adiabatic processes: So, statement (3) gives us:
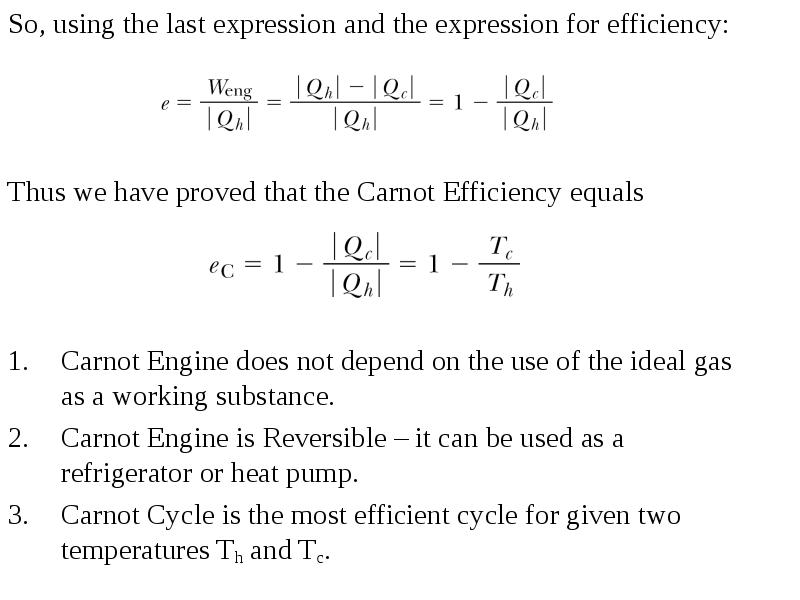
- 14. So, using the last expression and the expression for efficiency: So,
- 15. Carnot theorem The Carnot engine is the most efficient engine possible
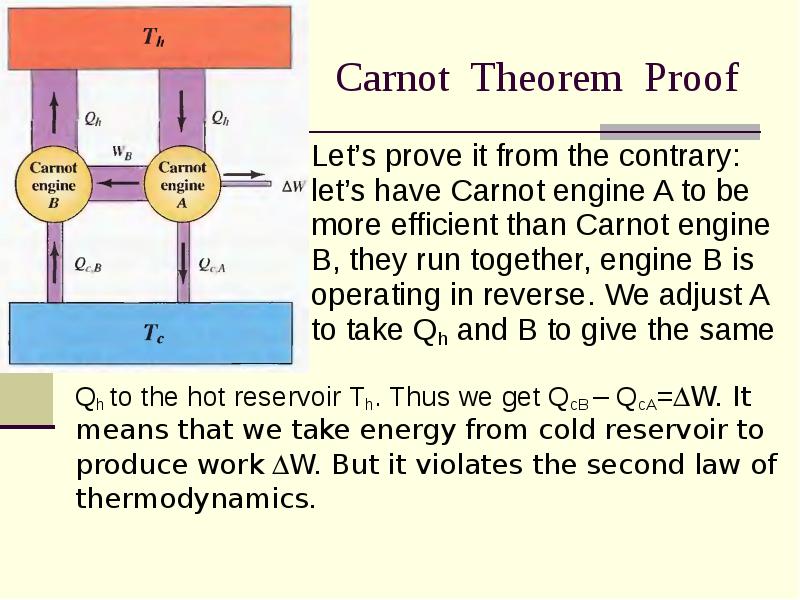
- 16. Carnot Theorem Proof
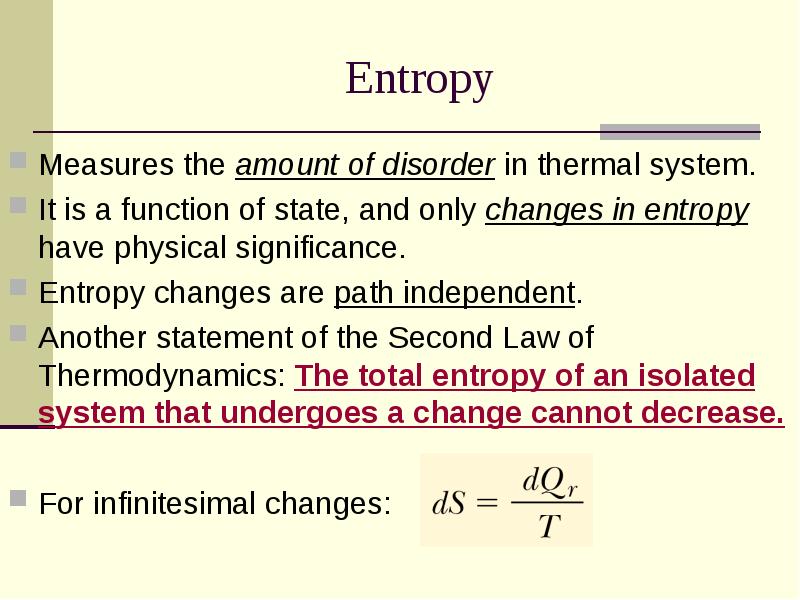
- 17. Entropy Measures the amount of disorder in thermal system. It is
- 18. Entropy change calculations Entropy is a state variable, the change in
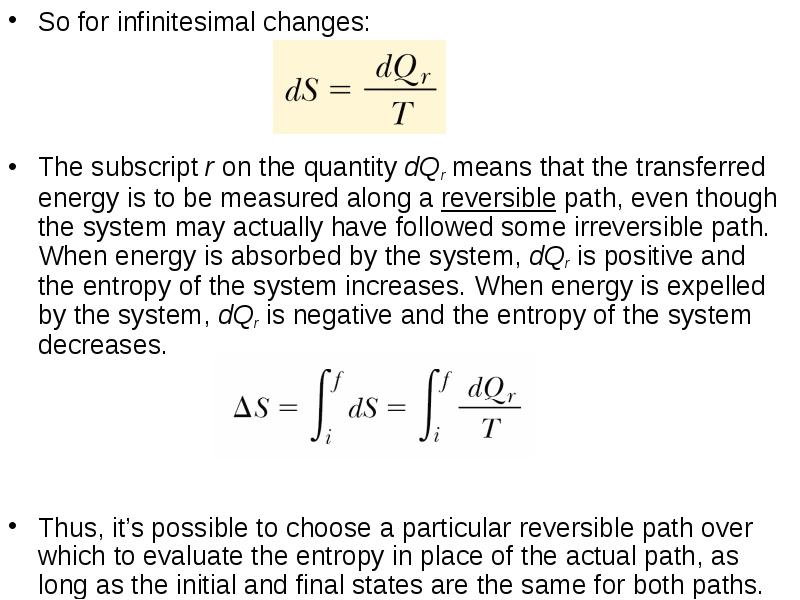
- 19. So for infinitesimal changes: So for infinitesimal changes: The subscript r
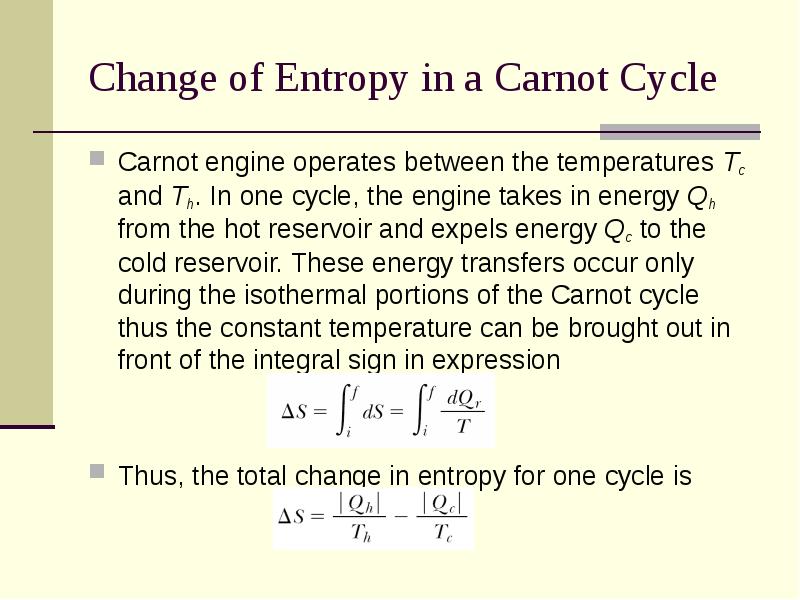
- 20. Change of Entropy in a Carnot Cycle Carnot engine operates between
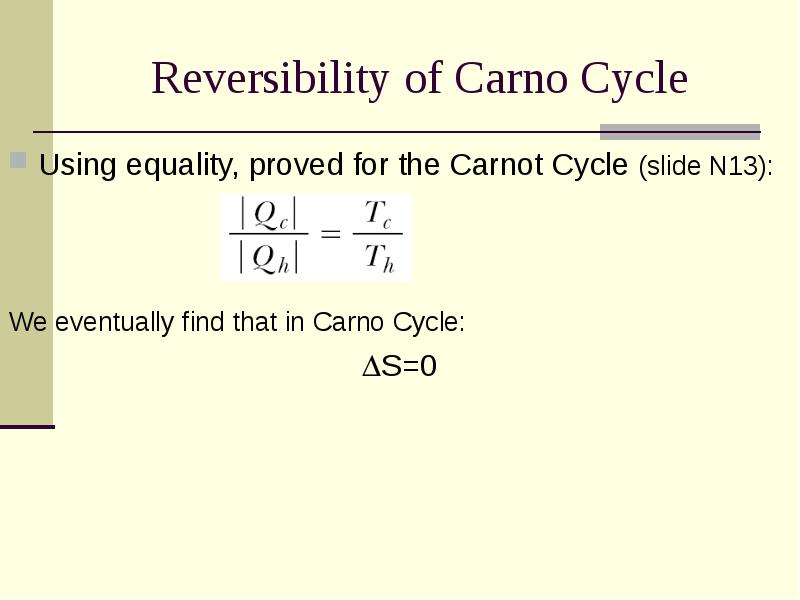
- 21. Reversibility of Carno Cycle Using equality, proved for the Carnot Cycle
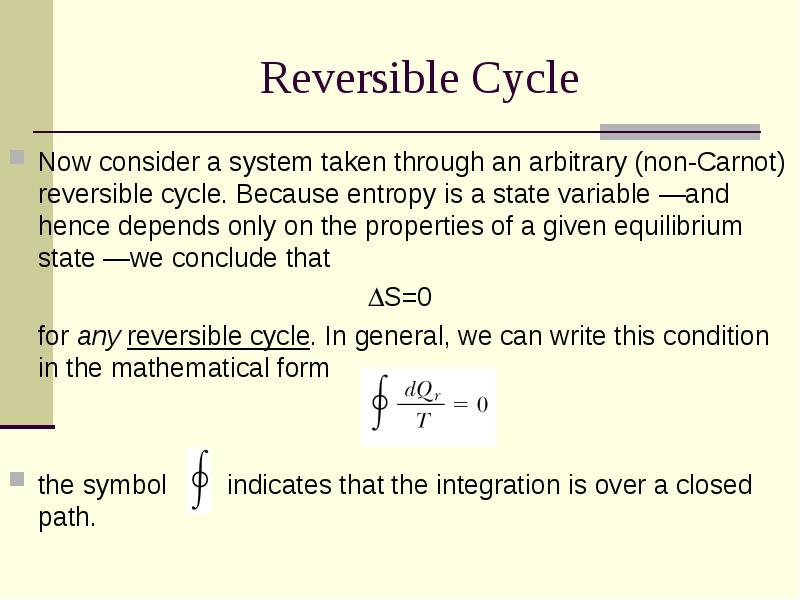
- 22. Reversible Cycle Now consider a system taken through an arbitrary (non-Carnot)
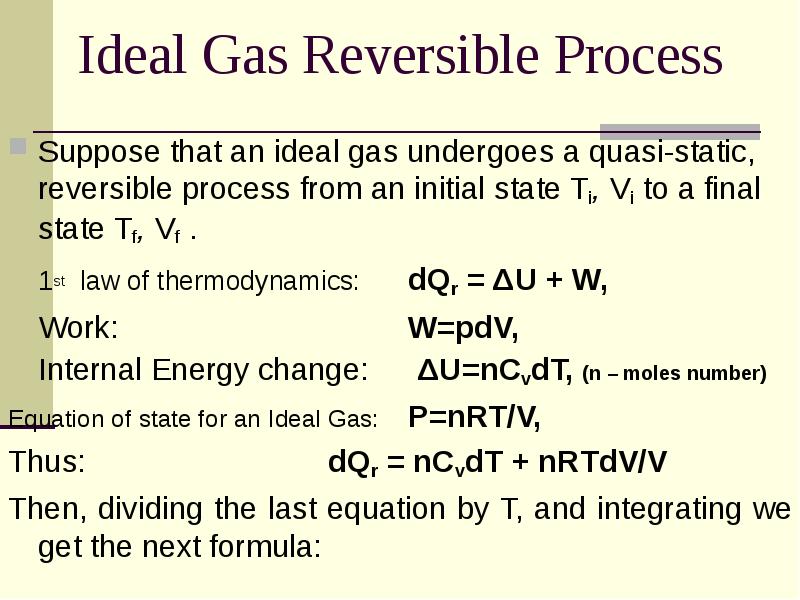
- 23. Ideal Gas Reversible Process Suppose that an ideal gas undergoes a
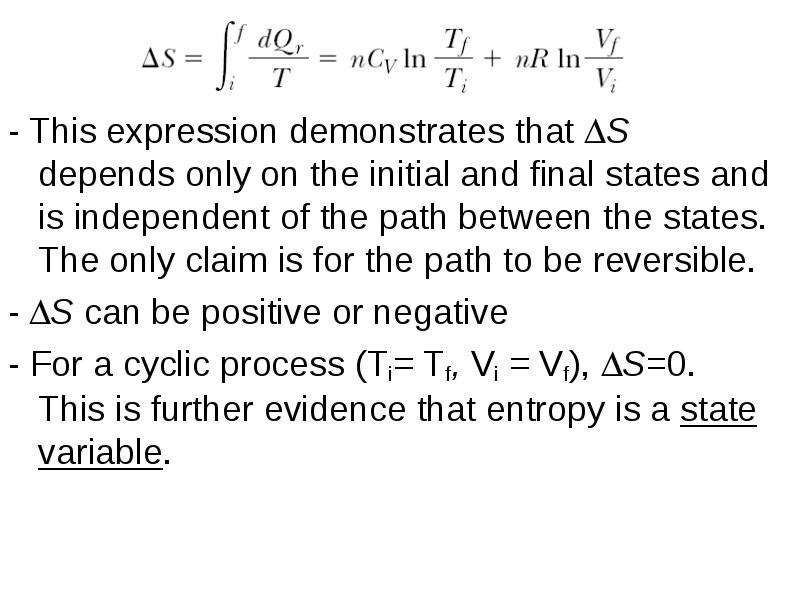
- 24. - This expression demonstrates that S depends only on the initial
- 25. The Second Law of Thermodynamics The total entropy of an isolated
- 26. Microscopic States Every macrostate can be realized by a number of
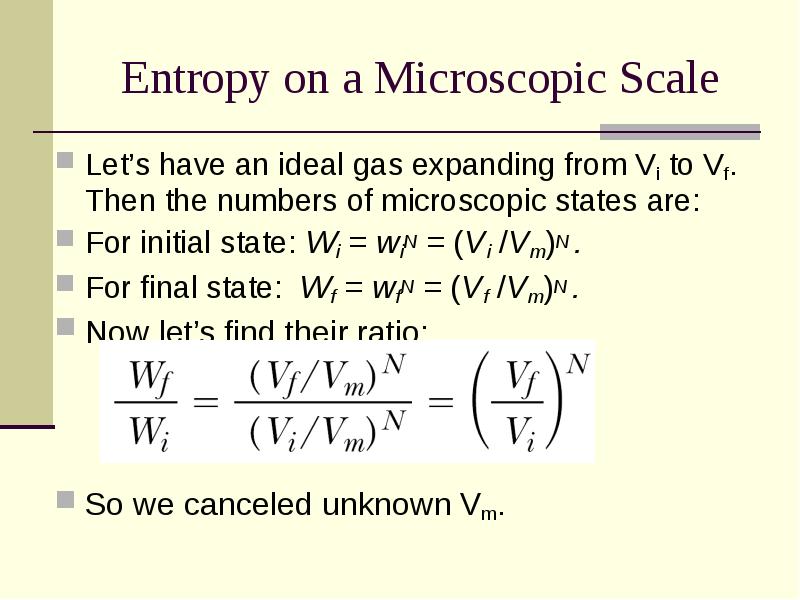
- 27. Entropy on a Microscopic Scale Let’s have an ideal gas expanding
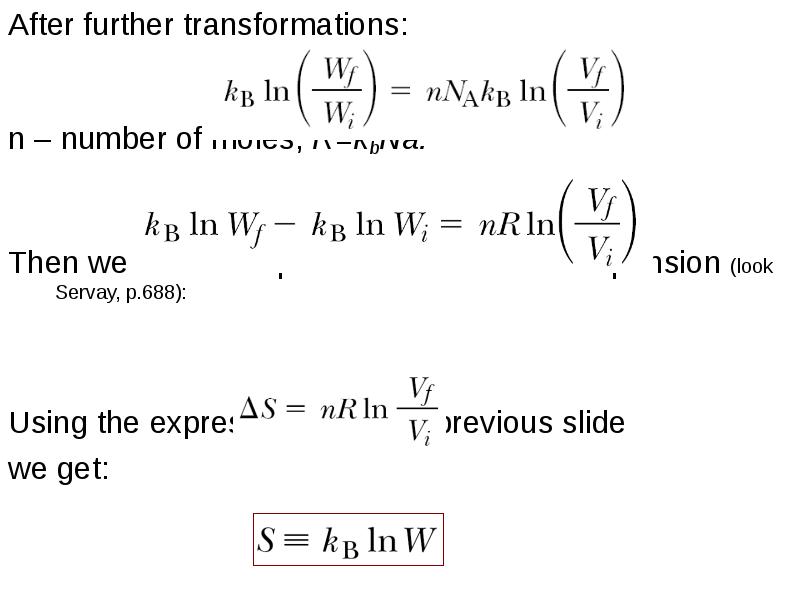
- 28. After further transformations: After further transformations: n – number of moles,
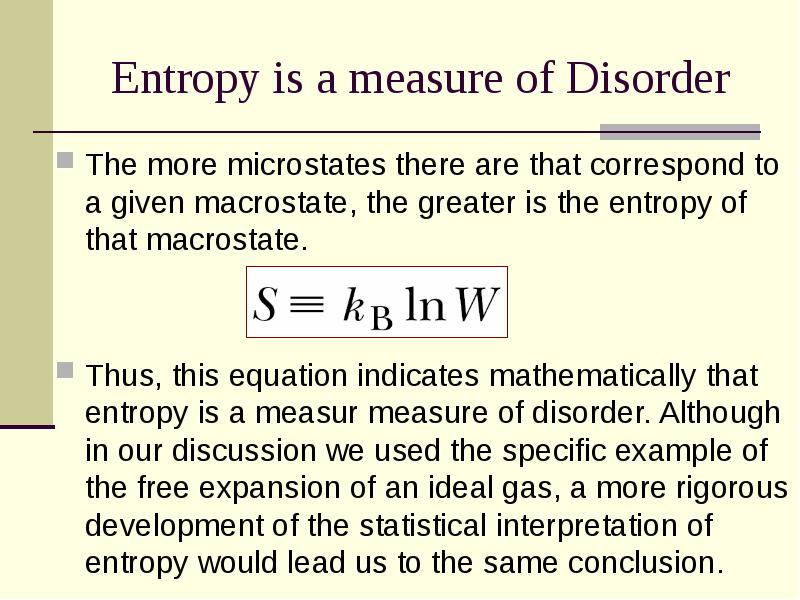
- 29. Entropy is a measure of Disorder The more microstates there are
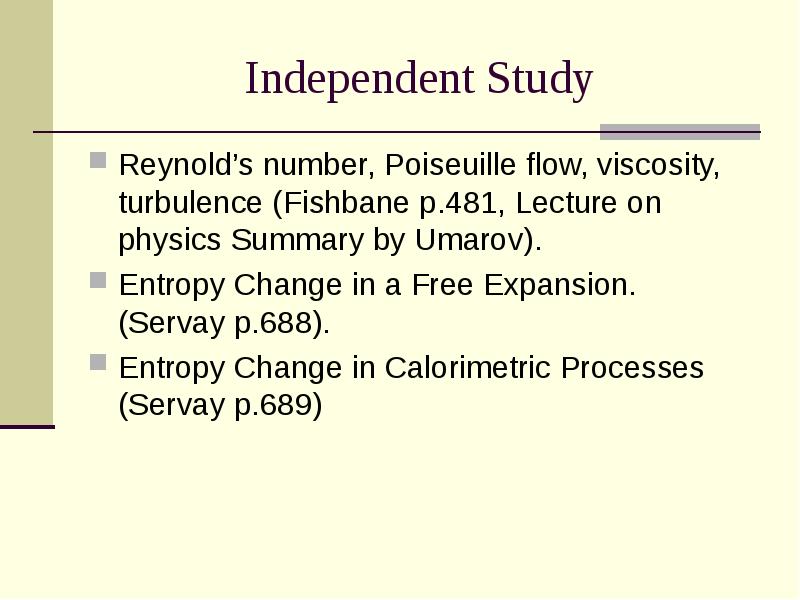
- 30. Independent Study Reynold’s number, Poiseuille flow, viscosity, turbulence (Fishbane p.481, Lecture
- 31. Скачать презентацию




Слайды и текст этой презентации
Похожие презентации