RHODIUM презентация
Содержание
- 3. Discovery and naming In the early 1800s, Wollaston was studying an
- 4. Physical properties Rhodium is a silver-white metal. It has a melting
- 5. Chemical properties Rhodium is a relatively inactive metal. It is not
- 6. Occurrence in nature Rhodium is one of the rarest elements on
- 7. Isotopes Only one naturally occurring isotope of rhodium is known, rhodium-103. Rhodium also
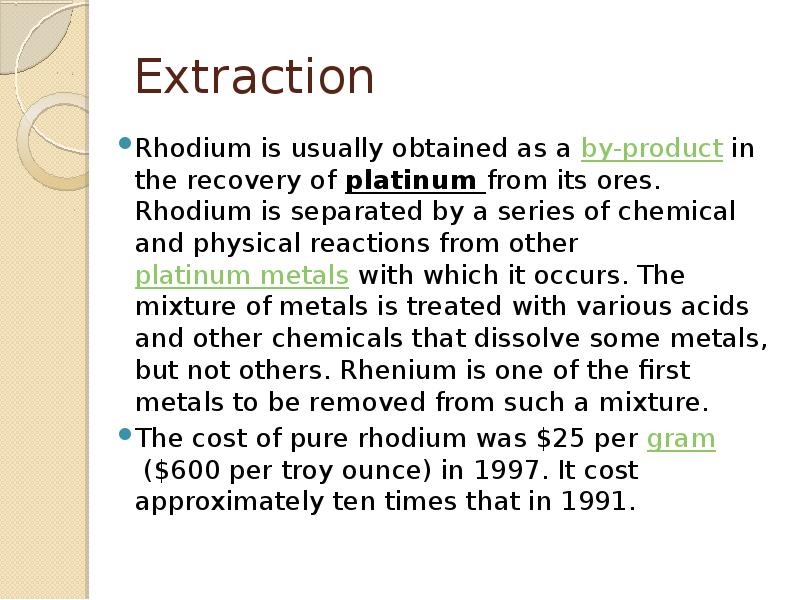
- 8. Extraction Rhodium is usually obtained as a by-product in the recovery of platinum from its
- 9. Uses Most of the rhodium metal sold in the United States
- 12. Compounds Compounds of rhodium are used as catalysts. A catalyst is
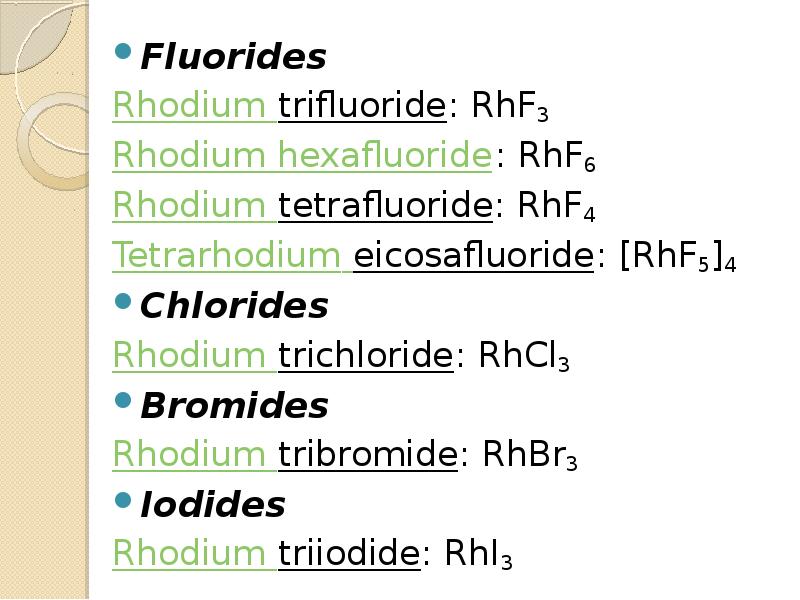
- 13. Fluorides Fluorides Rhodium trifluoride: RhF3 Rhodium hexafluoride: RhF6 Rhodium tetrafluoride: RhF4
- 14. Sulfides Sulfides Rhodium disulphide: RhS2 Dirhodium trisulphide: Rh2S3 Selenides Rhodium diselenide:
- 15. Health effects There are no studies of the health effects from
- 16. THANKS FOR YOUR ATTENTION!!! THANKS FOR YOUR ATTENTION!!!
- 17. Скачать презентацию














Слайды и текст этой презентации
Похожие презентации





























