Redox Reactions презентация
Содержание
- 2. Combustion, explosions, rusting, rotting, breathing
- 3. Oxidation numbers The oxidation state or oxidation number, is an indicator
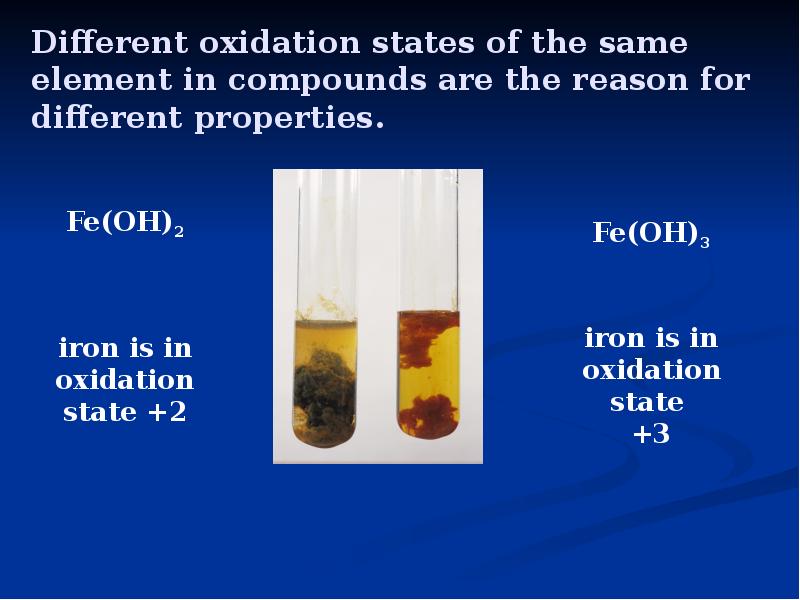
- 4. Different oxidation states of the same element in compounds are the
- 5. Plutonium oxidation states
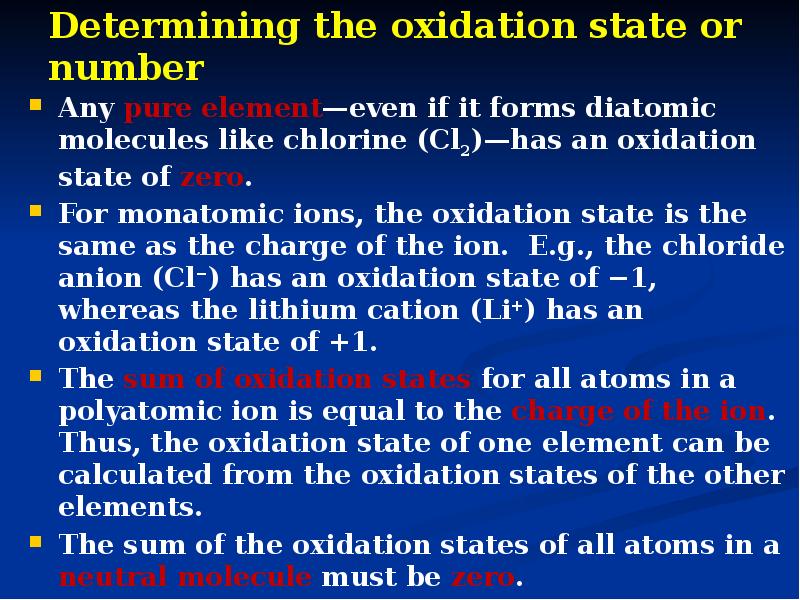
- 6. Determining the oxidation state or number Any pure element—even if
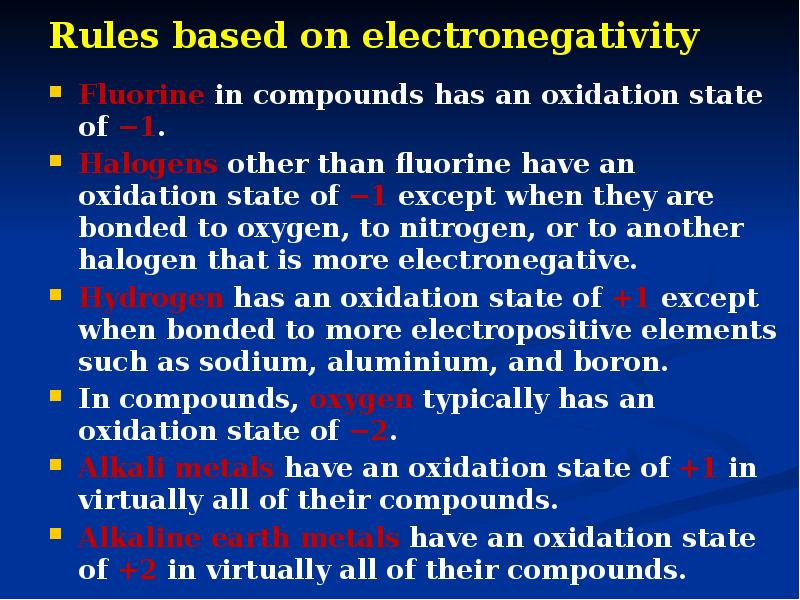
- 7. Rules based on electronegativity Fluorine in compounds has an oxidation
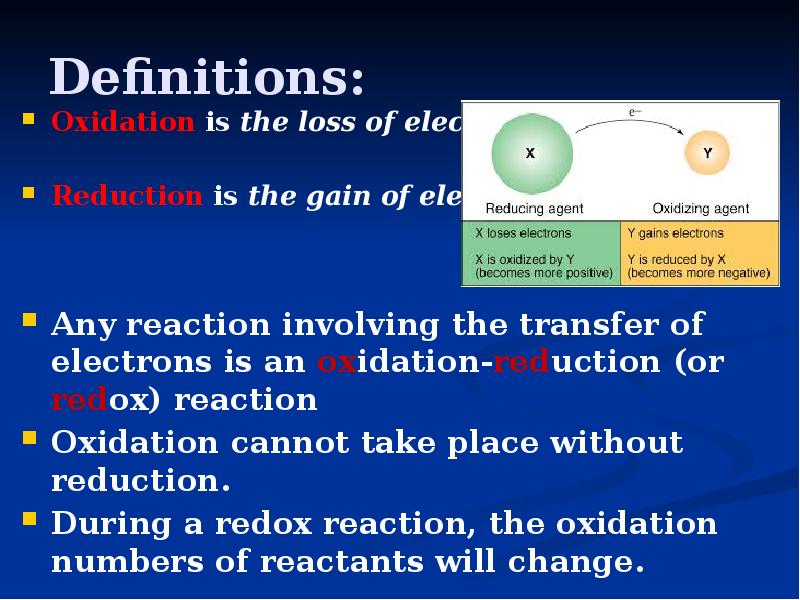
- 8. Definitions: Oxidation is the loss of electrons. Reduction is the
- 9. Oxidizing agents Substances that have the ability to oxidize other substances
- 10. In the Thermit reaction, shown here, which substance is reduced and
- 11. Reducing agents Substances that have the ability to reduce other substances
- 12. For any equation to be balanced: 1. The number of atoms
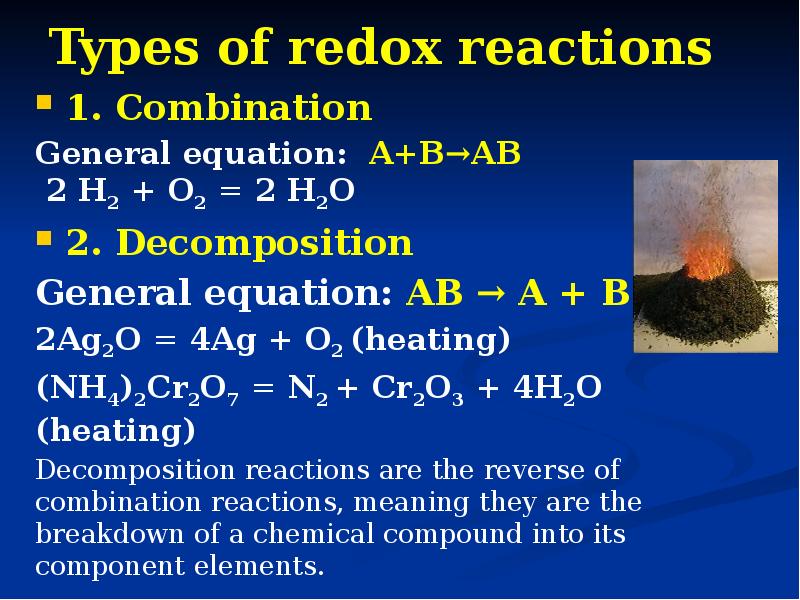
- 13. Types of redox reactions 1. Combination General equation: A+B→AB 2 H2
- 14. Balancing the equations K+Mn+7O4-2 + K+Cl- + H2SO4 = Cl20 +
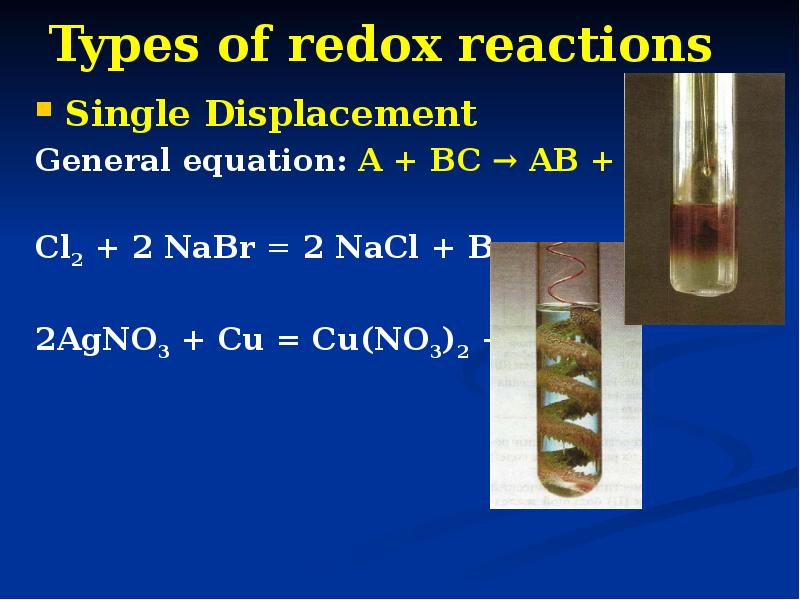
- 15. Types of redox reactions Single Displacement General equation: A + BC
- 16. Types of redox reactions Combustion CH₄ + 2O₂ → CO₂+ 2H₂O
- 17. Types of redox reactions Disproportionation The same substances are both oxidized
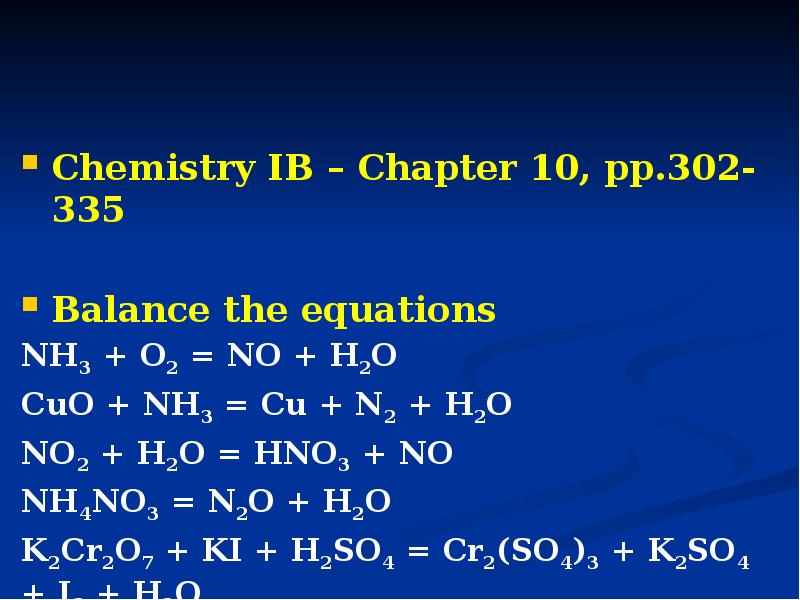
- 18. Chemistry IB – Chapter 10, pp.302-335 Balance the equations NH3
- 19. Скачать презентацию











Слайды и текст этой презентации
Похожие презентации





























