Colligative properties of solutions презентация
Содержание
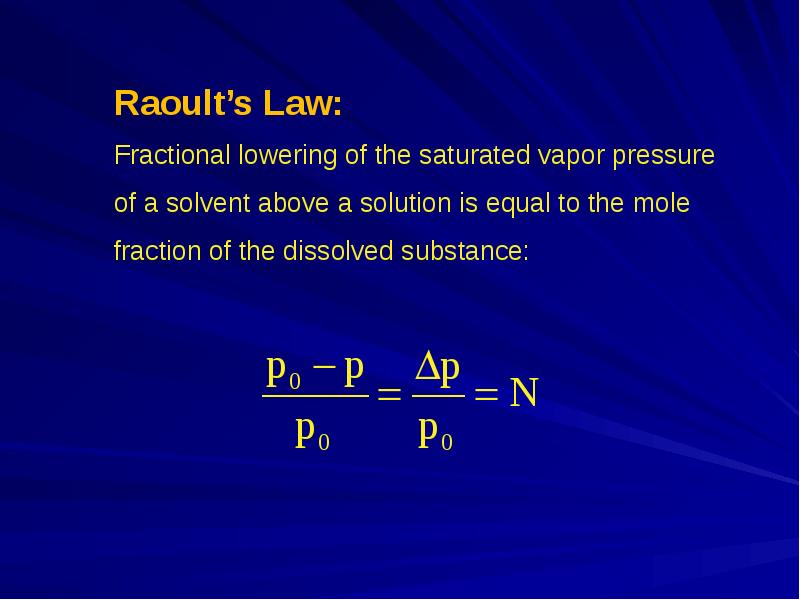
- 2. Properties of solutions that depend on the number of molecules present
- 5. Spontaneous process of solute concentration leveling in the whole volume of
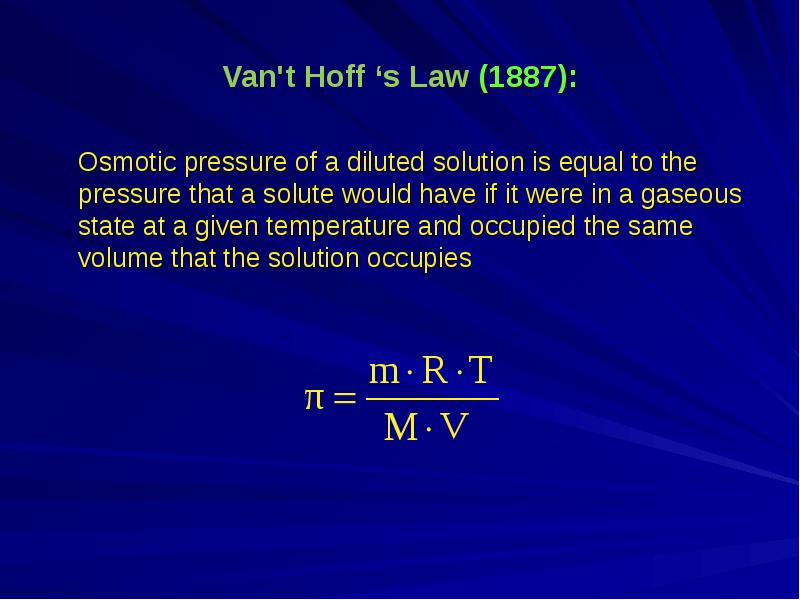
- 7. Van't Hoff ‘s Law (1887): Osmotic pressure of a diluted solution
- 8. Turgor is a state of tension of the cellular cover caused
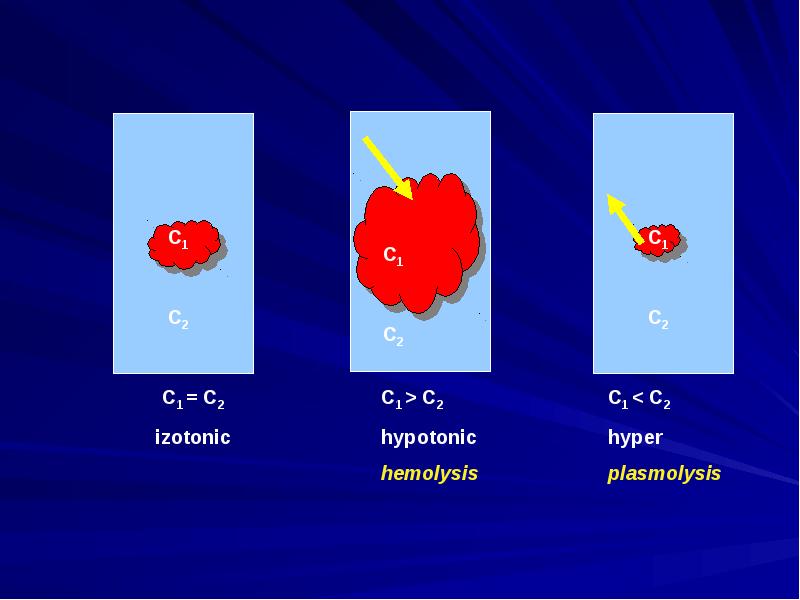
- 9. Solutions with an identical osmotic pressure are called isotonic. Solutions
- 11. Скачать презентацию







Слайды и текст этой презентации
Скачать презентацию на тему Colligative properties of solutions можно ниже:
Похожие презентации