Molecular-kinetic theory of ideal gases презентация
Содержание
- 2. Lecture 5 MOLECULAR-KINETIC THEORY OF IDEAL GASES THE MOLECULAR BASIS
- 3. Main assumptions for Ideal Gas Model The number of molecules in
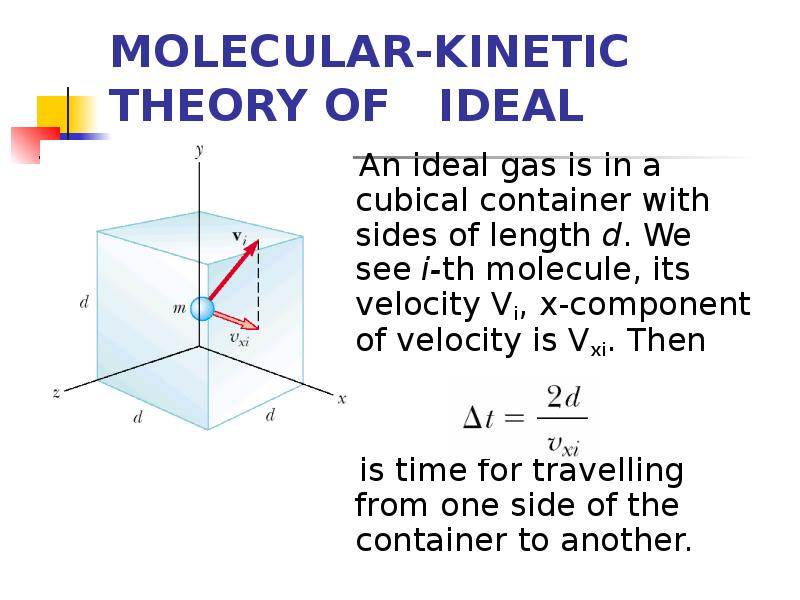
- 4. MOLECULAR-KINETIC THEORY OF IDEAL GASES An ideal gas is in
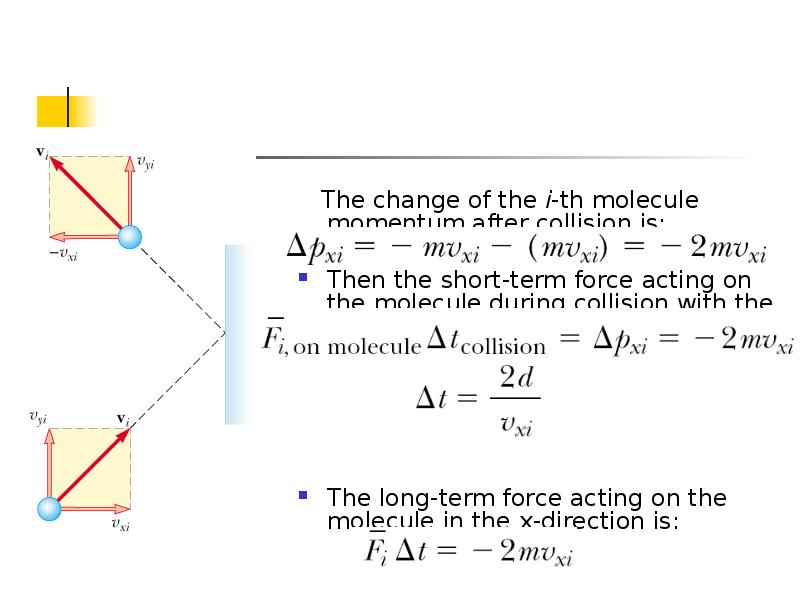
- 5. The change of the i-th molecule momentum after collision is: Then
- 6. Using previous expressions we can find x-component of the long-term average
- 7. Root mean square (rms) value of x-component of velocities of N
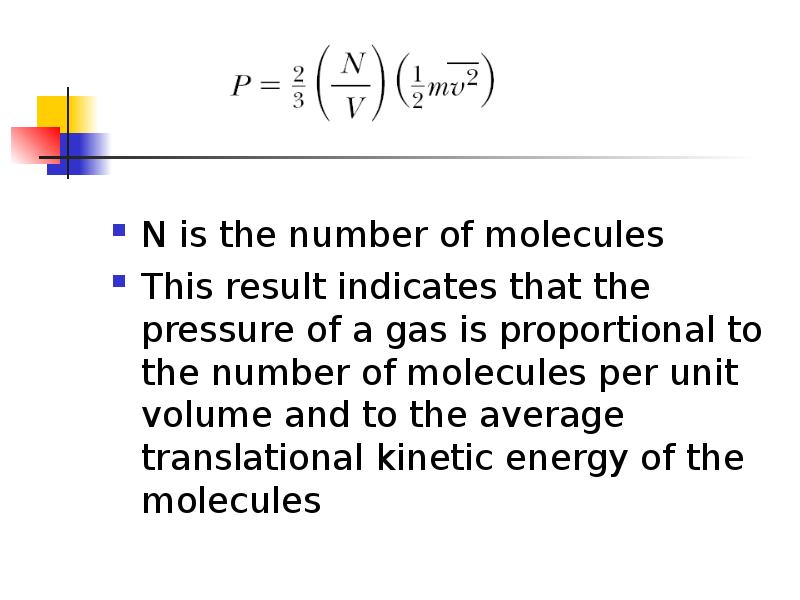
- 8. N is the number of molecules This result indicates that the
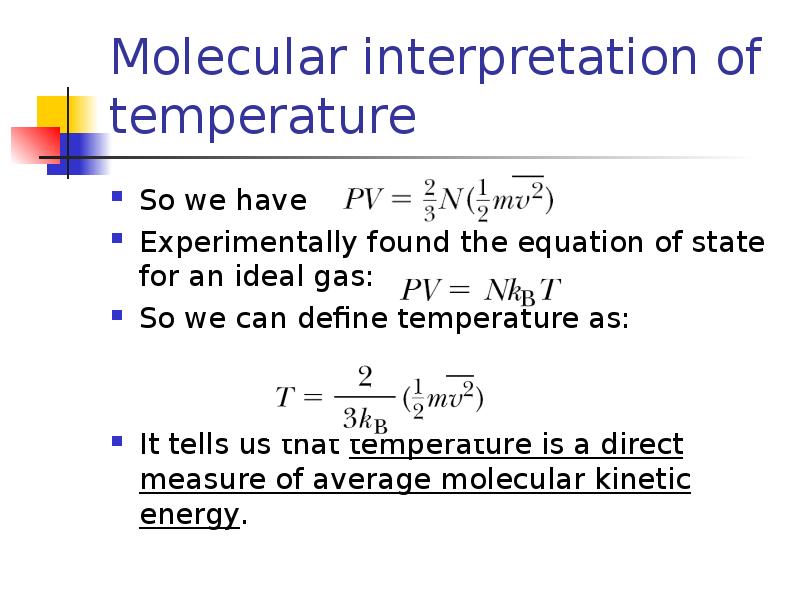
- 9. Molecular interpretation of temperature So we have Experimentally found the equation
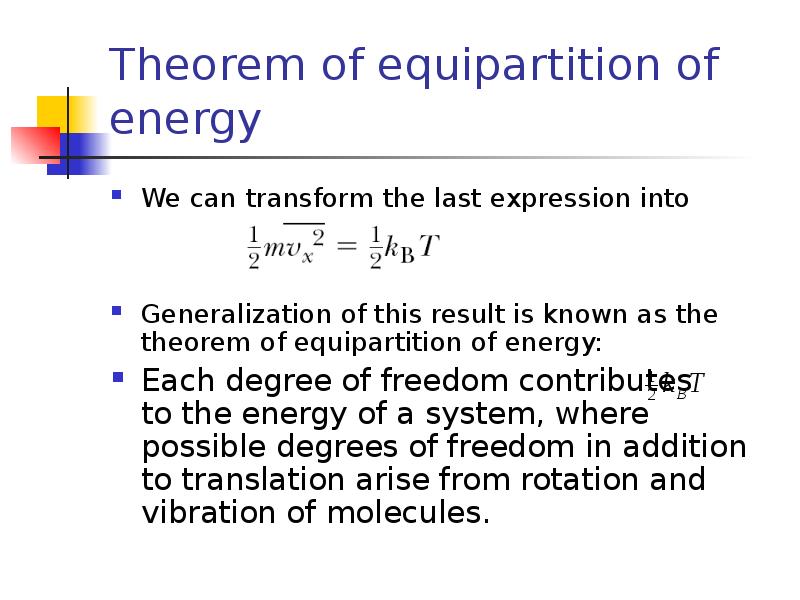
- 10. Theorem of equipartition of energy We can transform the last expression
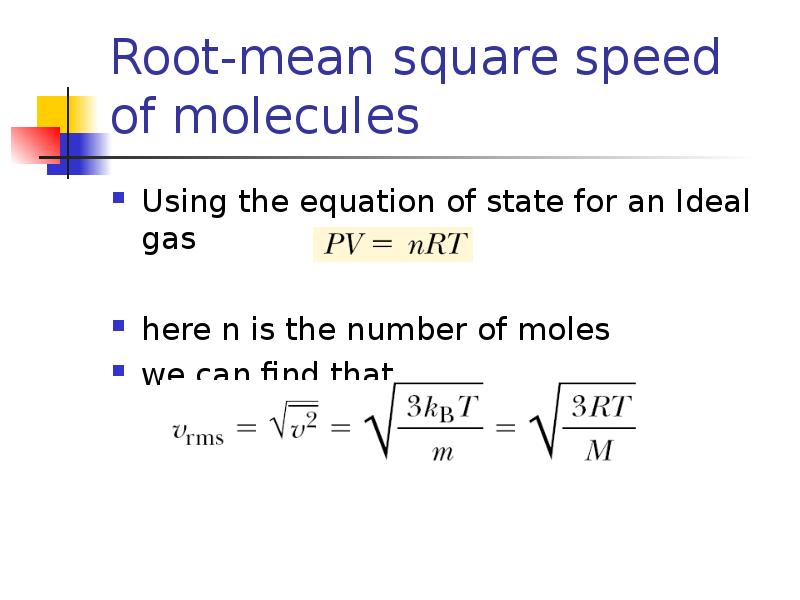
- 11. Root-mean square speed of molecules Using the equation of state for
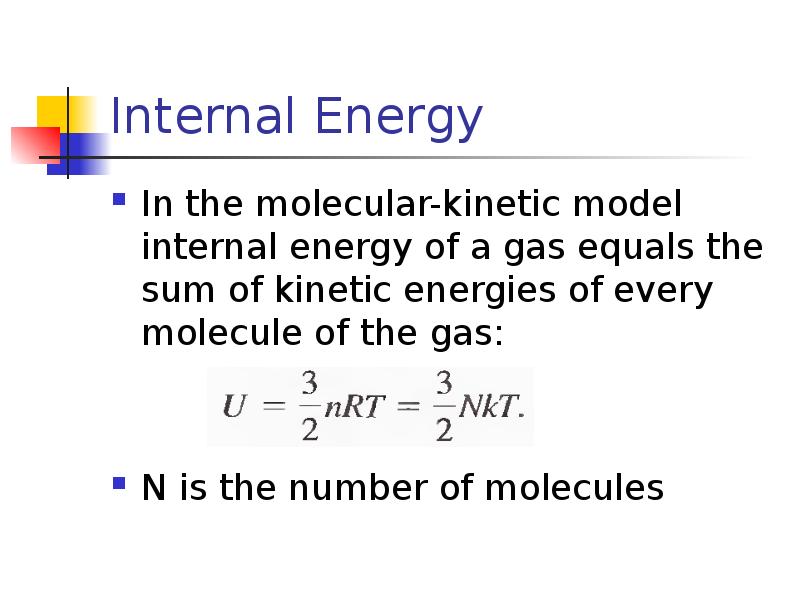
- 12. Internal Energy In the molecular-kinetic model internal energy of a gas
- 13. Equation of State for an Ideal Gas Found experimentally: n is
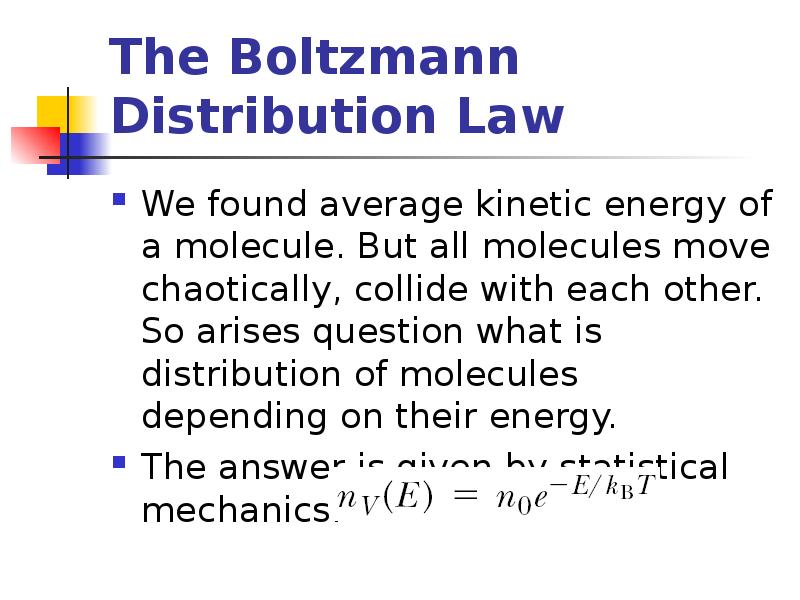
- 14. The Boltzmann Distribution Law We found average kinetic energy of a
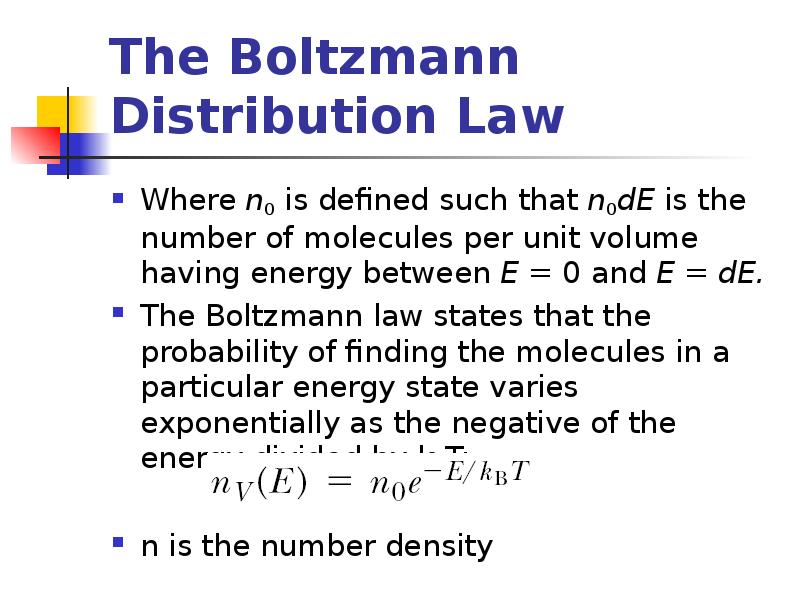
- 15. The Boltzmann Distribution Law Where n0 is defined such that n0dE
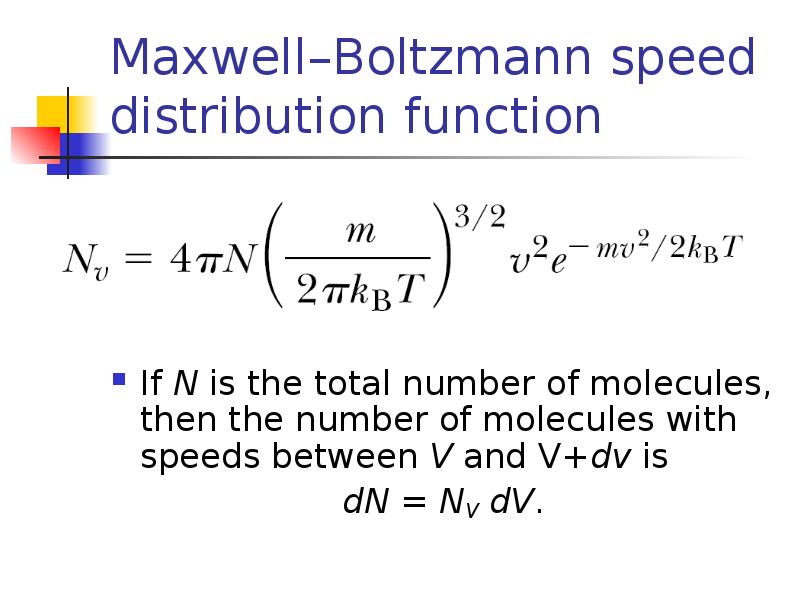
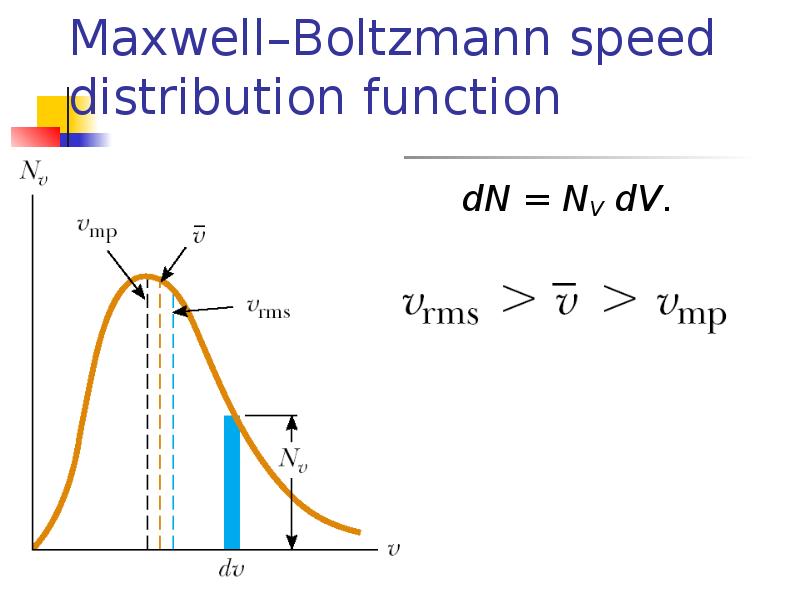
- 16. Maxwell–Boltzmann speed distribution function If N is the total number of
- 17. Maxwell–Boltzmann speed distribution function dN = NV dV.
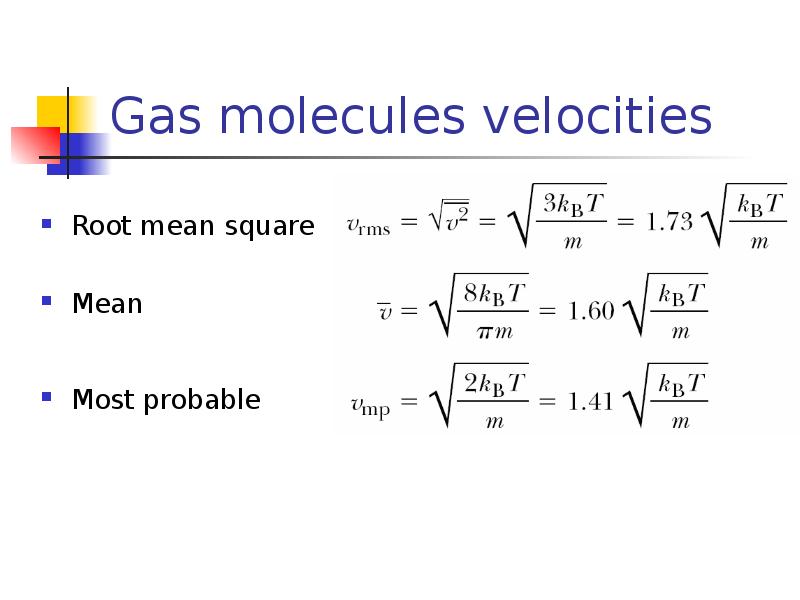
- 18. Gas molecules velocities Root mean square Mean Most probable
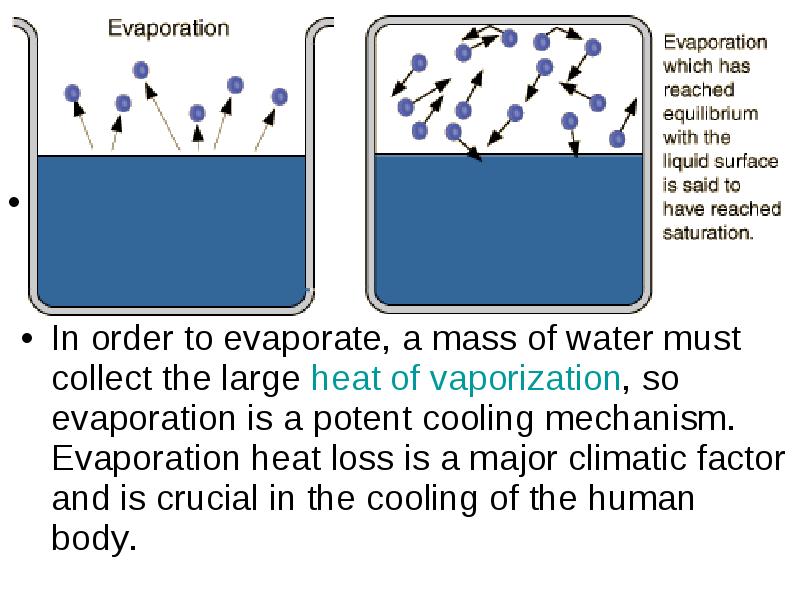
- 19. Evaporation We know that liquids evaporate when they’re below boiling temperature.
- 20. . .
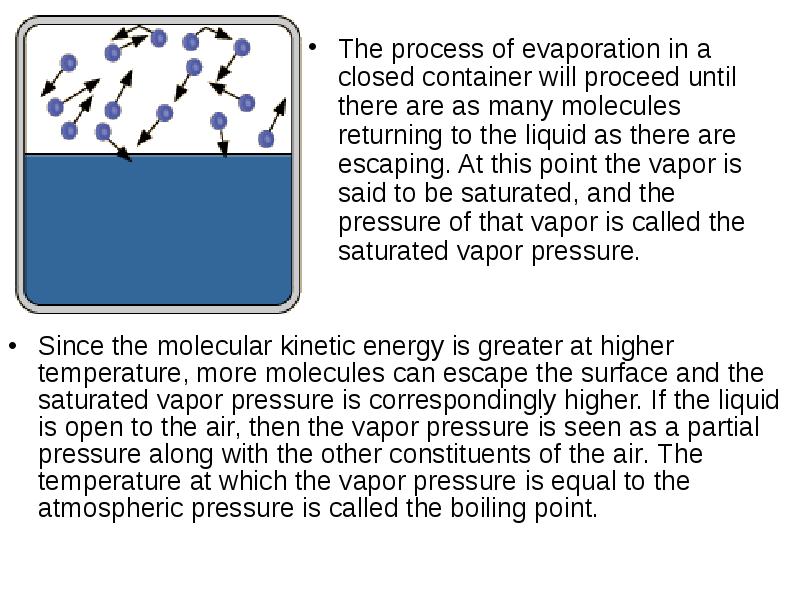
- 21. Saturation Vapor Pressure Ordinary evaporation is a surface phenomenon - some
- 22. The process of evaporation in a closed container will proceed until
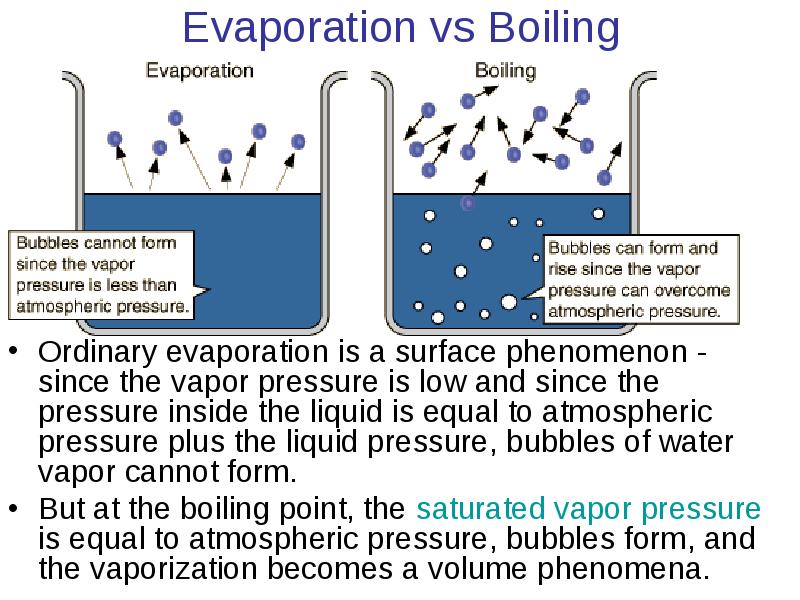
- 23. Evaporation vs Boiling Ordinary evaporation is a surface phenomenon -
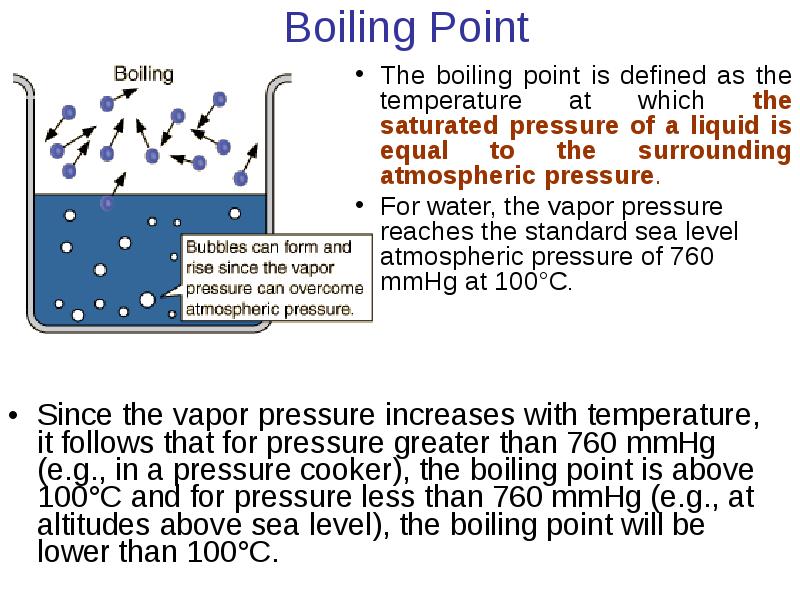
- 24. Boiling Point The boiling point is defined as the temperature at
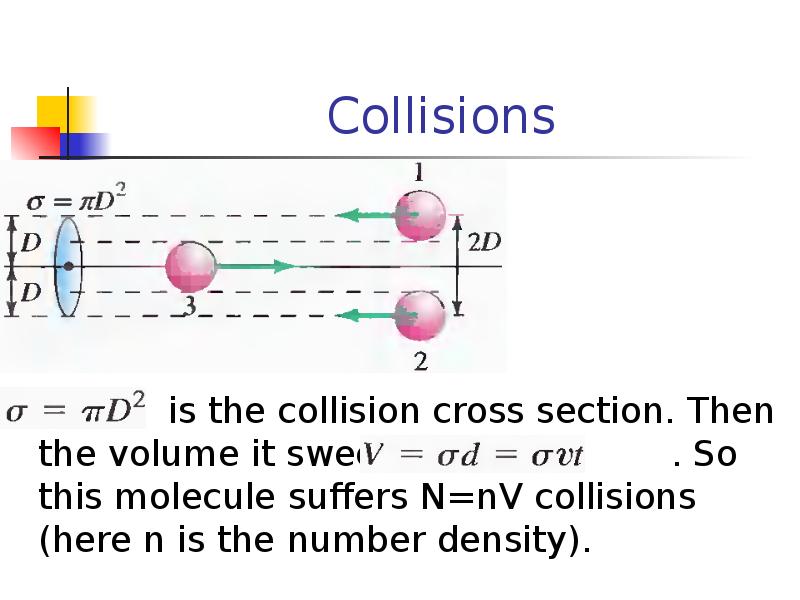
- 25. Collisions is the collision cross section. Then the volume it sweeps
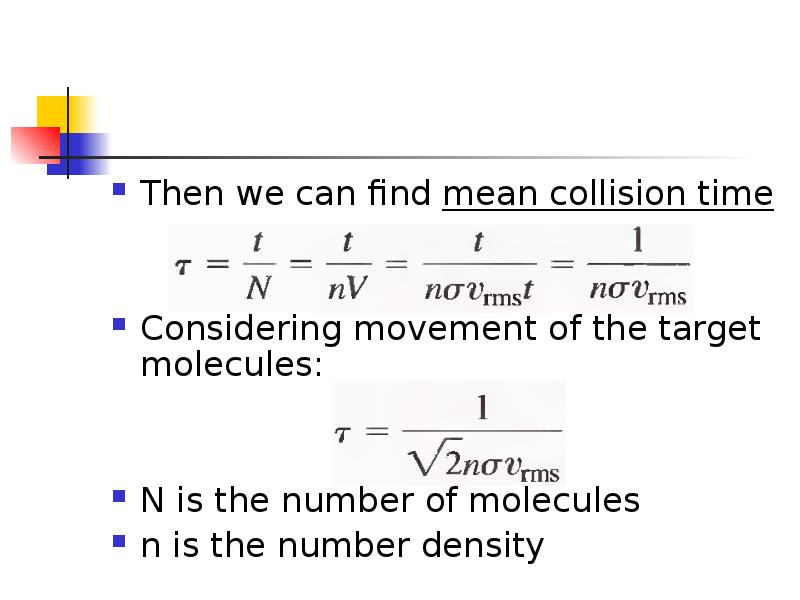
- 26. Then we can find mean collision time Considering movement of the
- 27. Mean free path is an average distance between collisions:
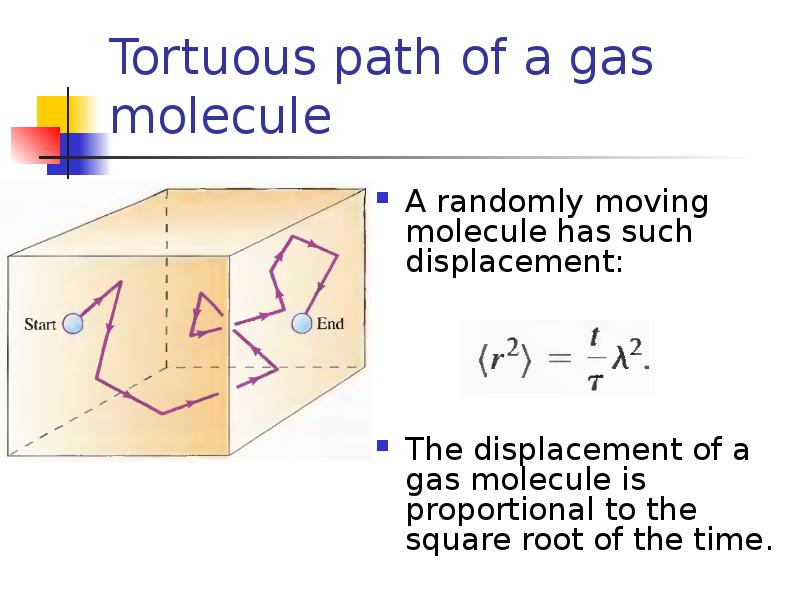
- 28. Tortuous path of a gas molecule A randomly moving molecule has
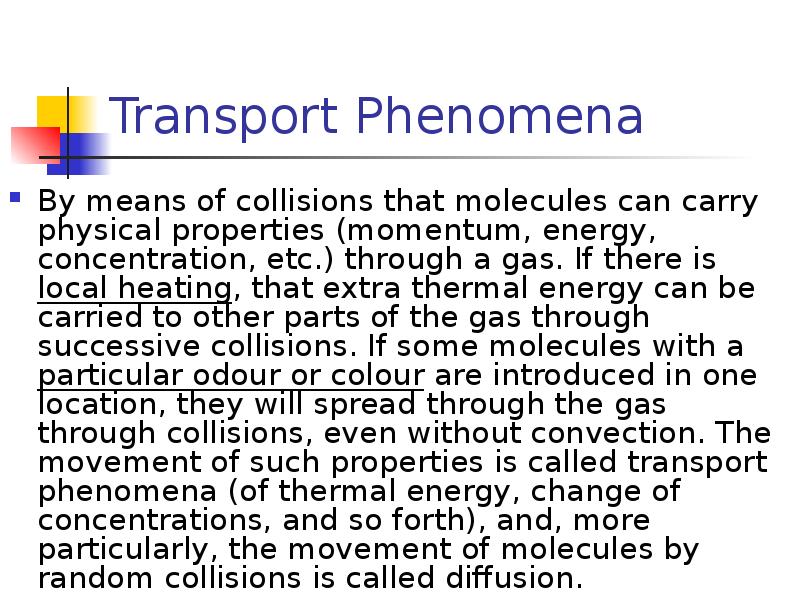
- 29. Transport Phenomena By means of collisions that molecules can carry physical
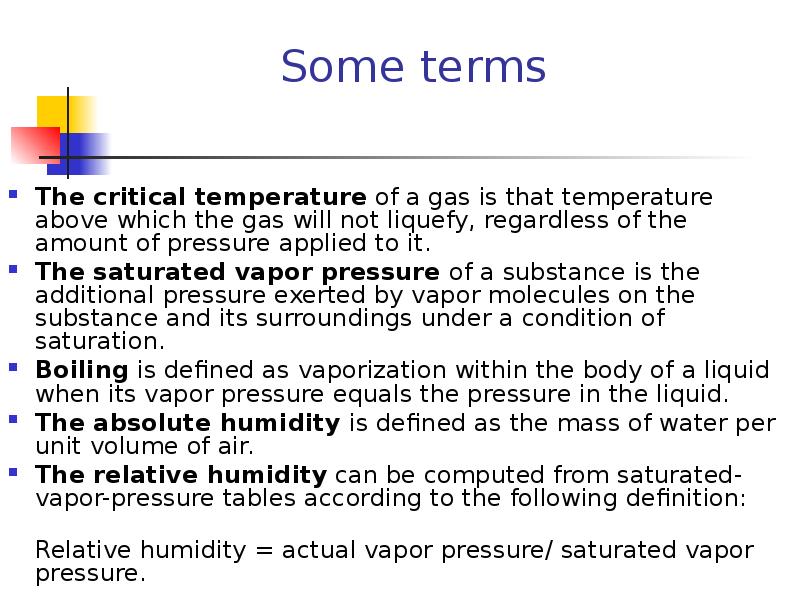
- 30. Some terms The critical temperature of a gas is that temperature
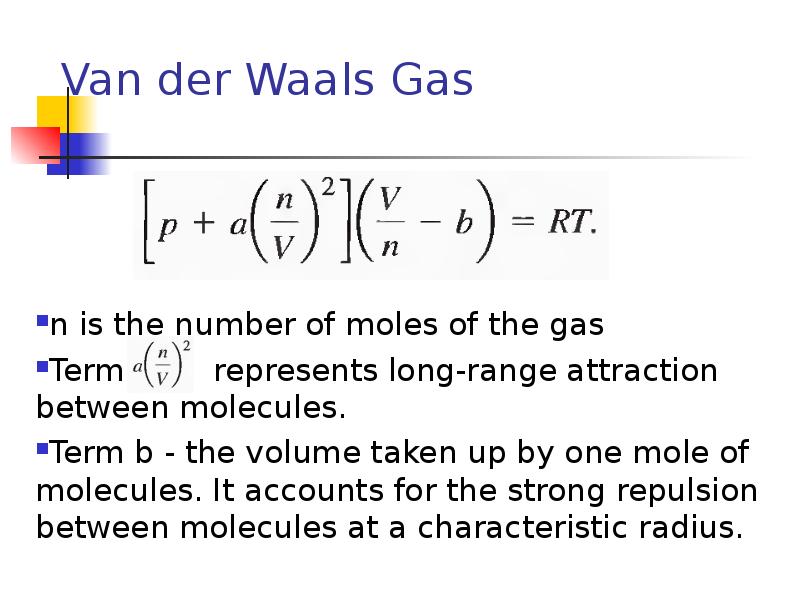
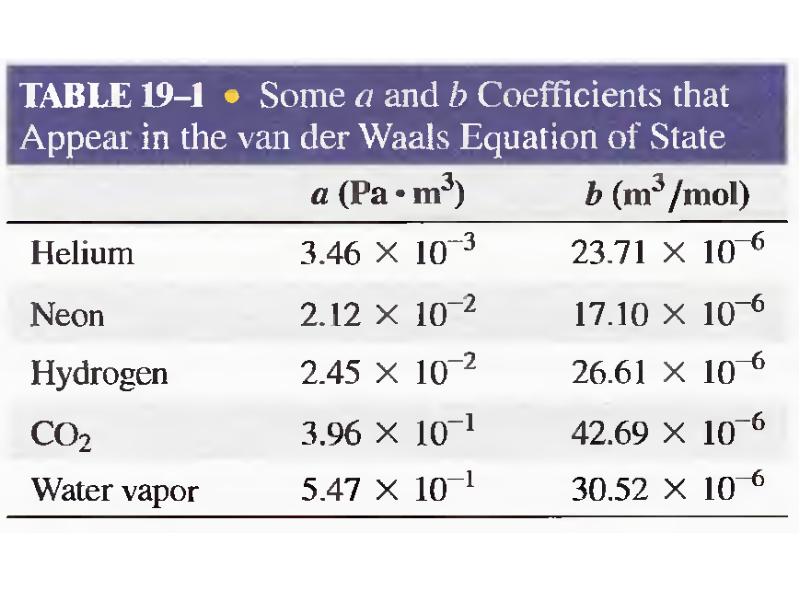
- 31. Van der Waals Gas n is the number of moles of
- 33. NOTE In this lecture quantity n is used in 2 different
- 34. Скачать презентацию



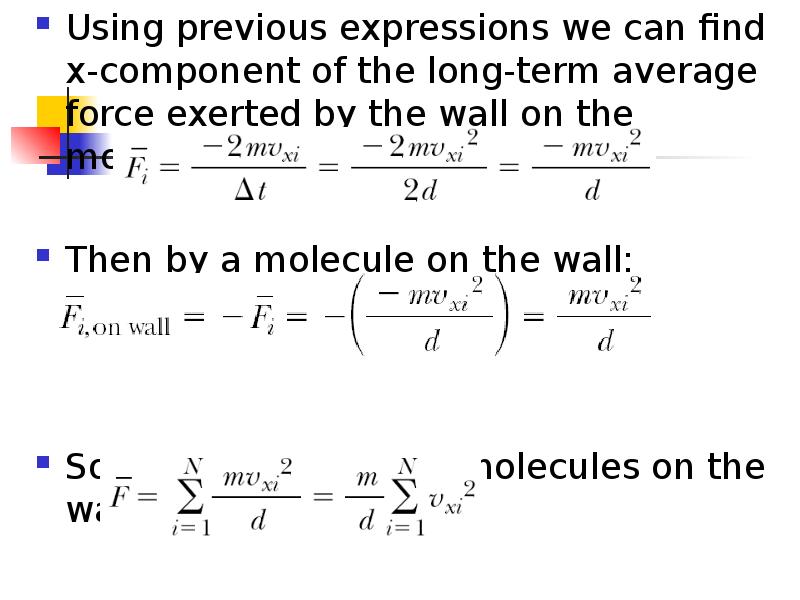
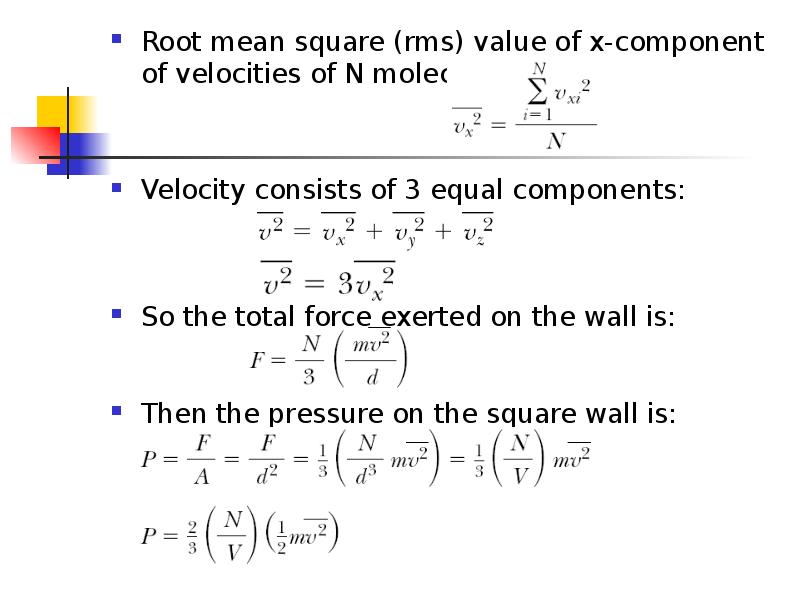





Слайды и текст этой презентации
Скачать презентацию на тему Molecular-kinetic theory of ideal gases можно ниже:
Похожие презентации