Nuclear magnetic resonance spectroscopy презентация
Содержание
- 2. SIMPLE PLAN ! THAT’S SO SIMPLE 1.Principles of molecular spectroscopy
- 3. Lets understand few things Electromagnetic Radiation – is propagated at
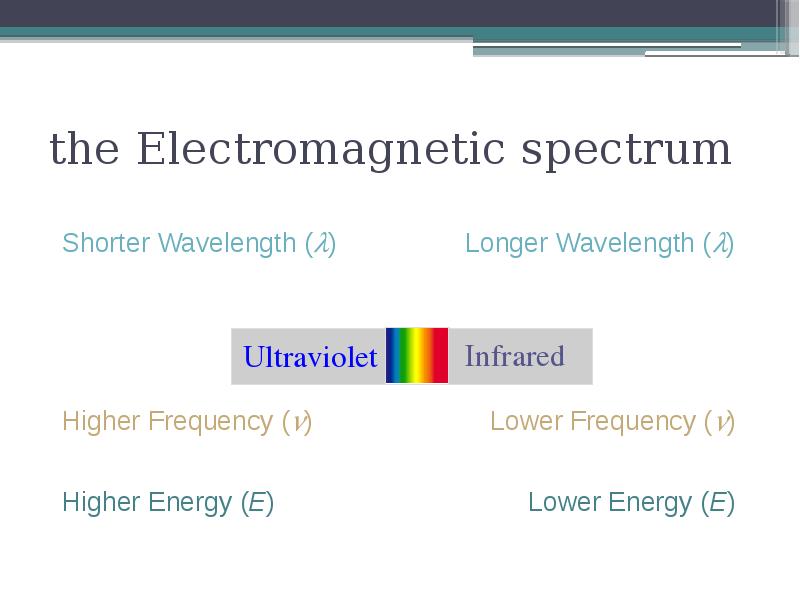
- 4. the Electromagnetic spectrum
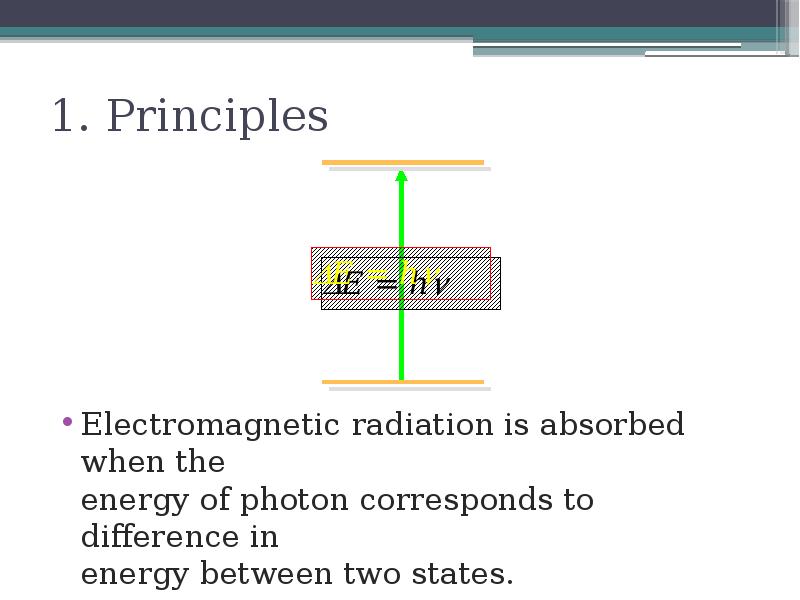
- 5. 1. Principles Electromagnetic radiation is absorbed when the energy of
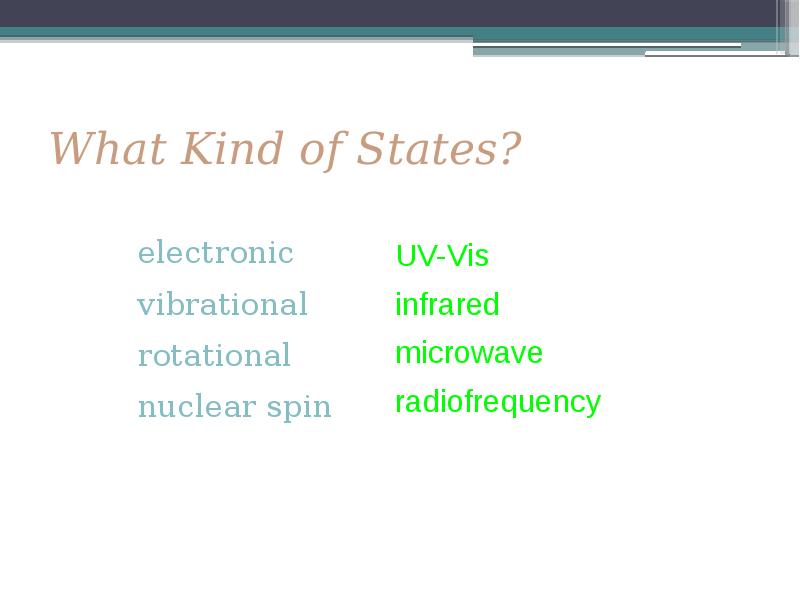
- 6. What Kind of States?
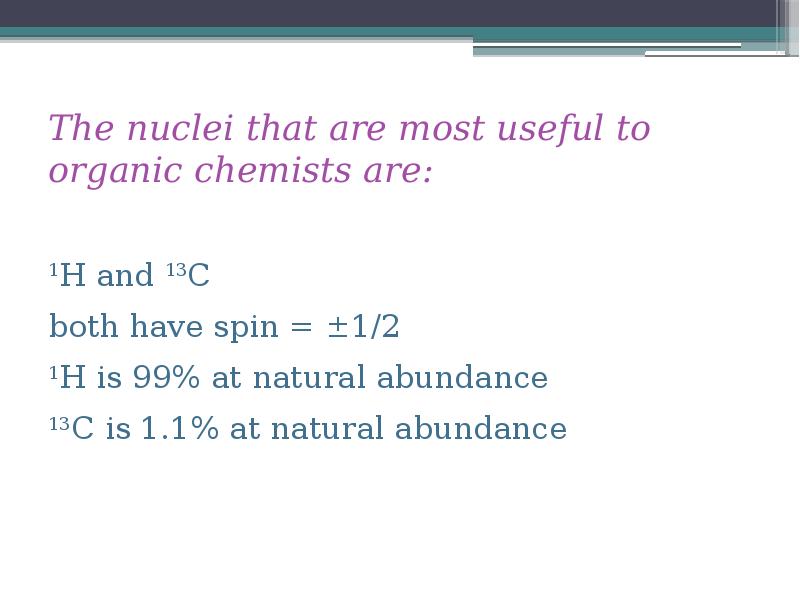
- 7. The nuclei that are most useful to organic chemists are: 1H
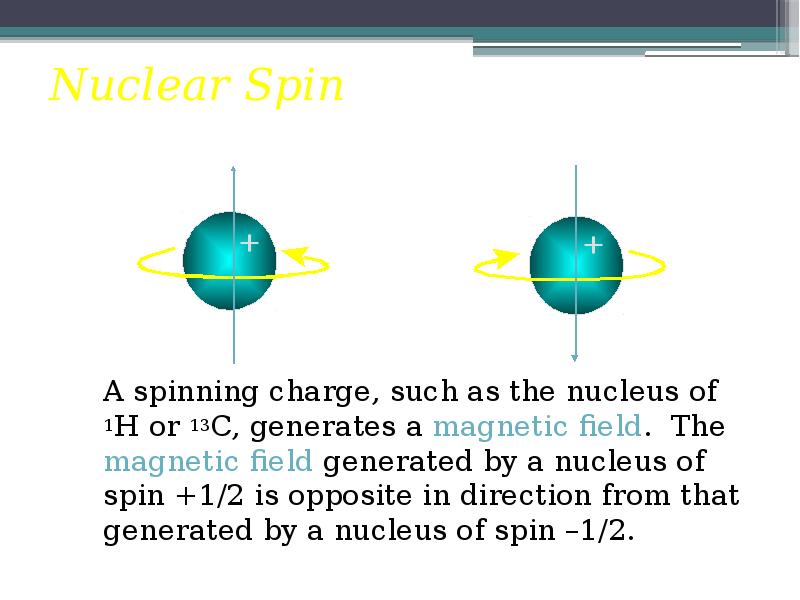
- 8. Nuclear Spin
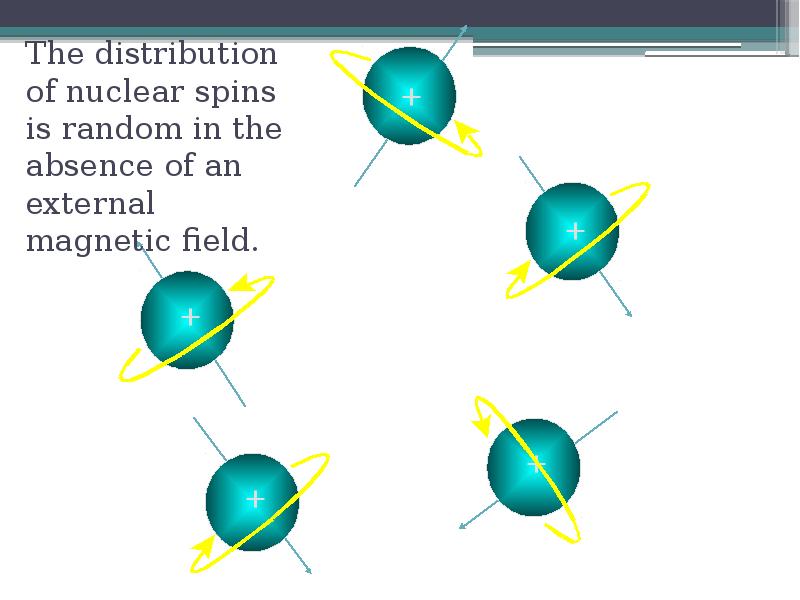
- 9. The distribution of nuclear spins is random in the absence of
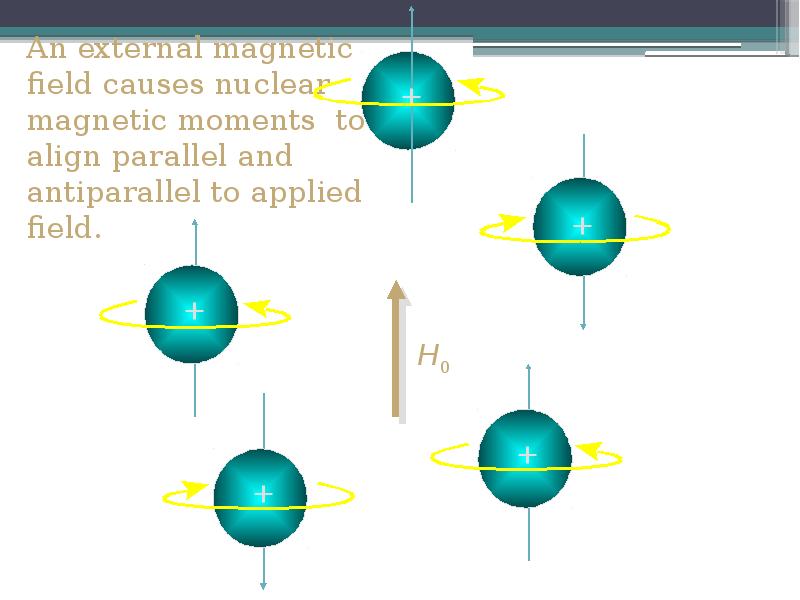
- 10. An external magnetic field causes nuclear magnetic moments to align parallel
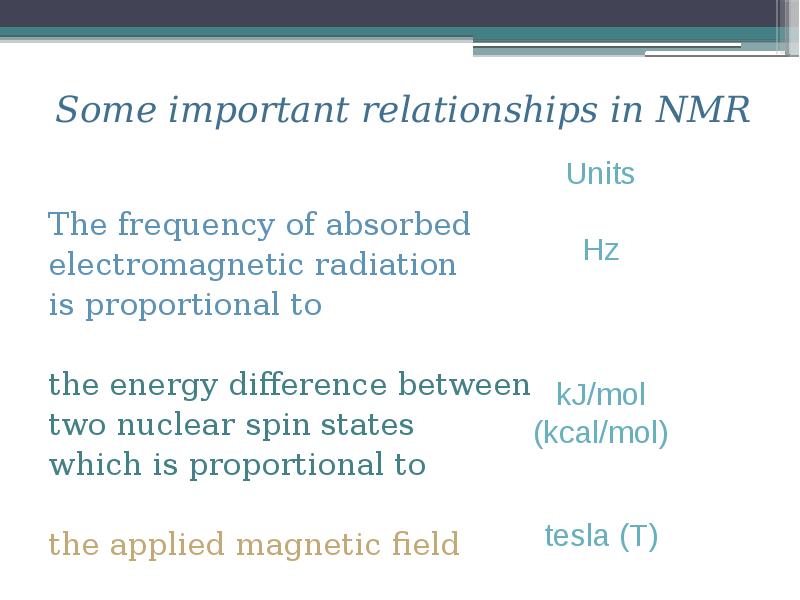
- 11. Some important relationships in NMR The frequency of absorbed electromagnetic radiation
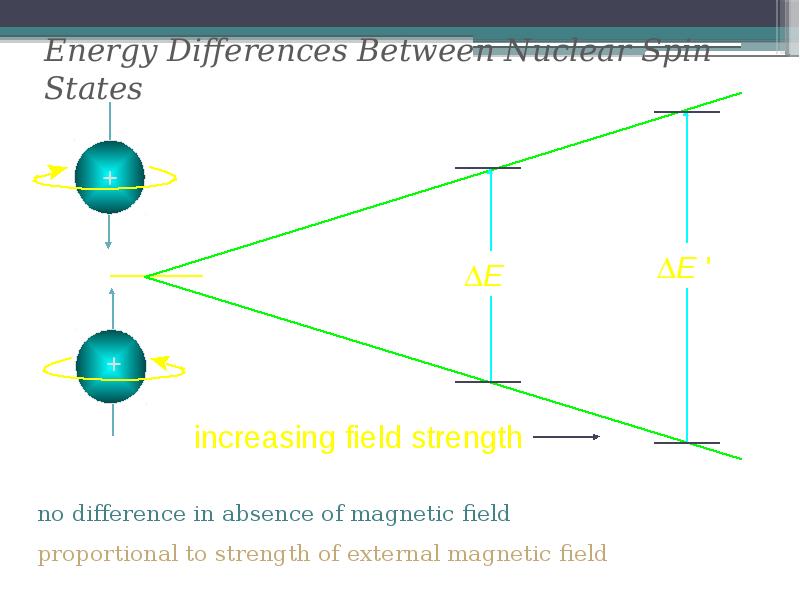
- 12. Energy Differences Between Nuclear Spin States
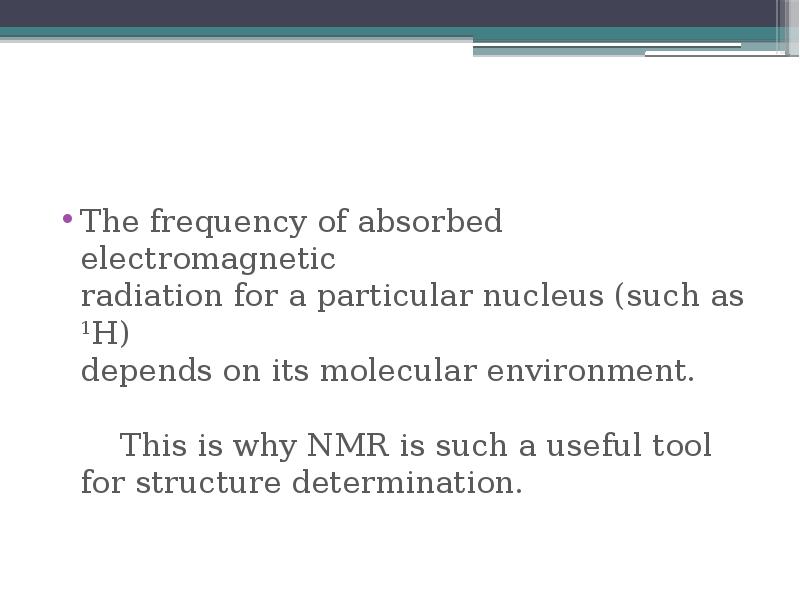
- 13. The frequency of absorbed electromagnetic radiation for a particular nucleus (such
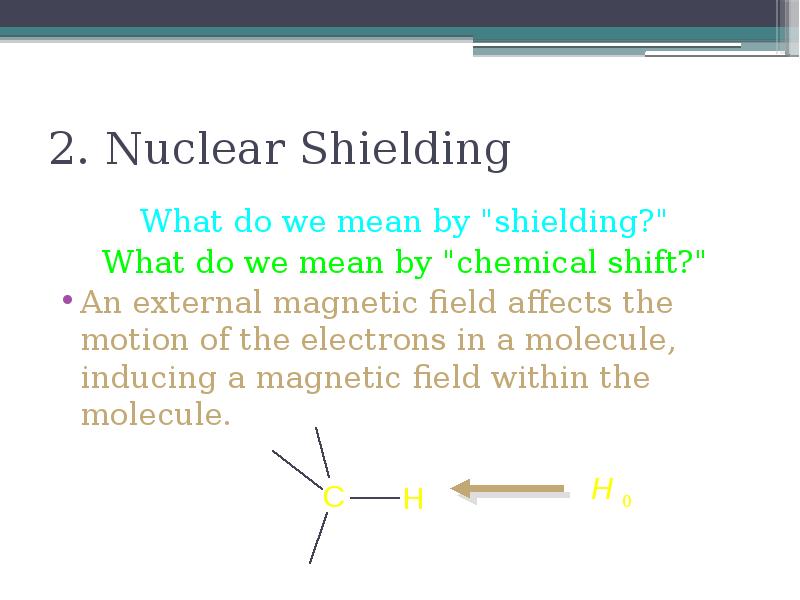
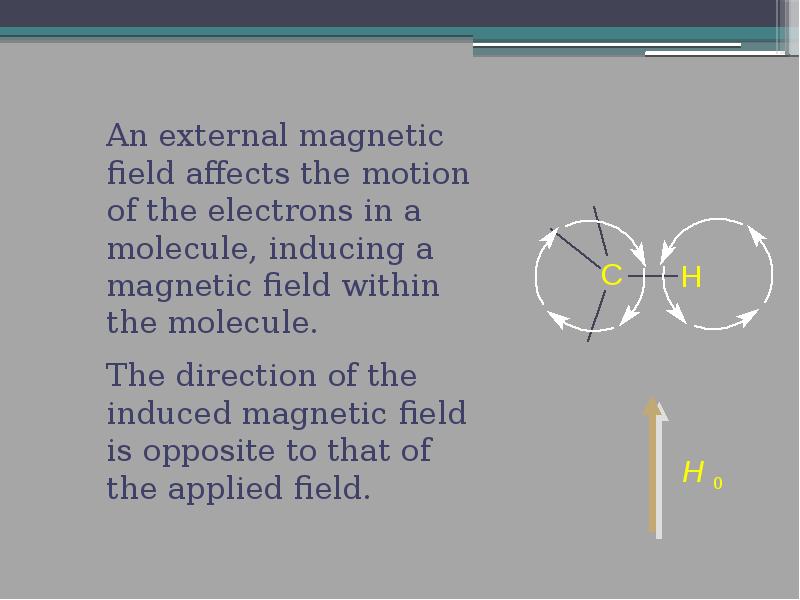
- 14. 2. Nuclear Shielding What do we mean by "shielding?" What do
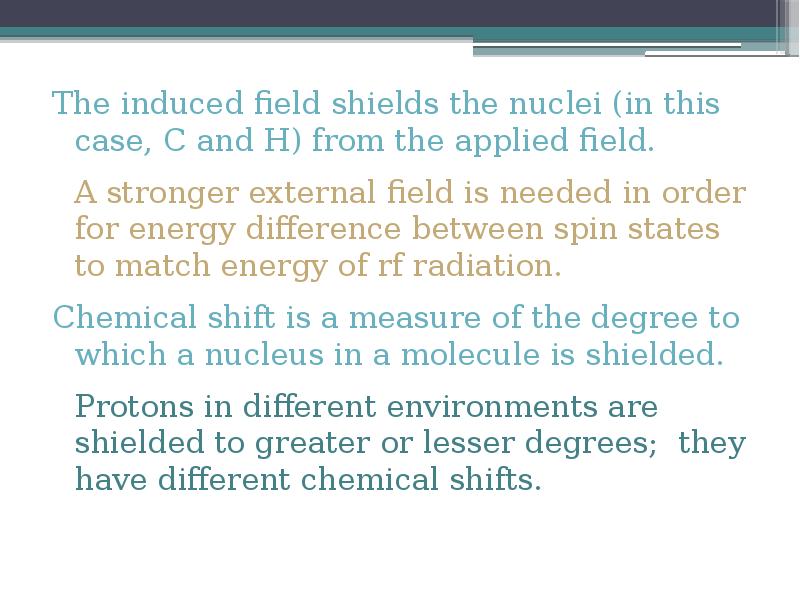
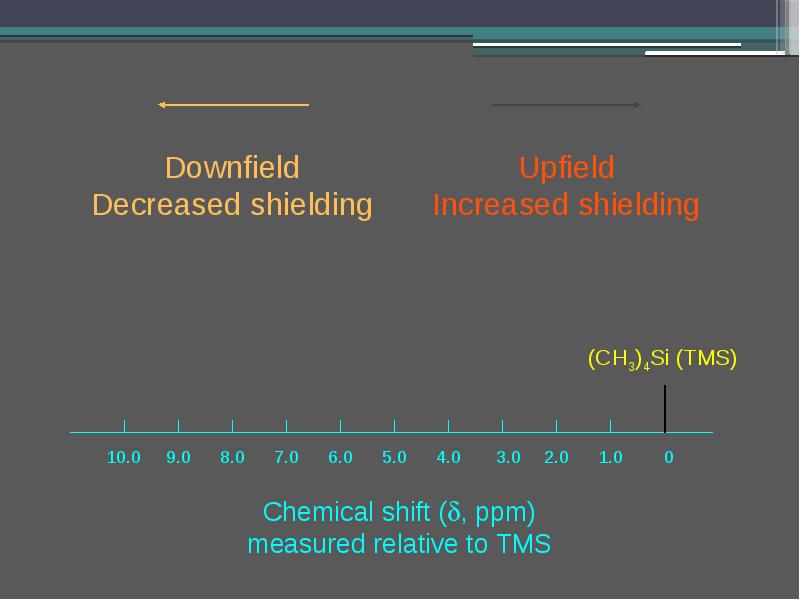
- 16. The induced field shields the nuclei (in this case, C and
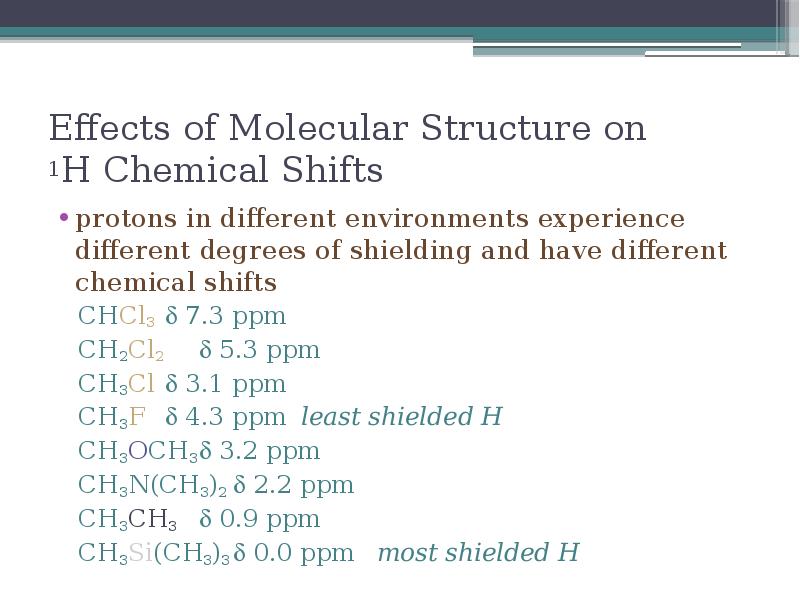
- 18. Effects of Molecular Structure on 1H Chemical Shifts protons in different
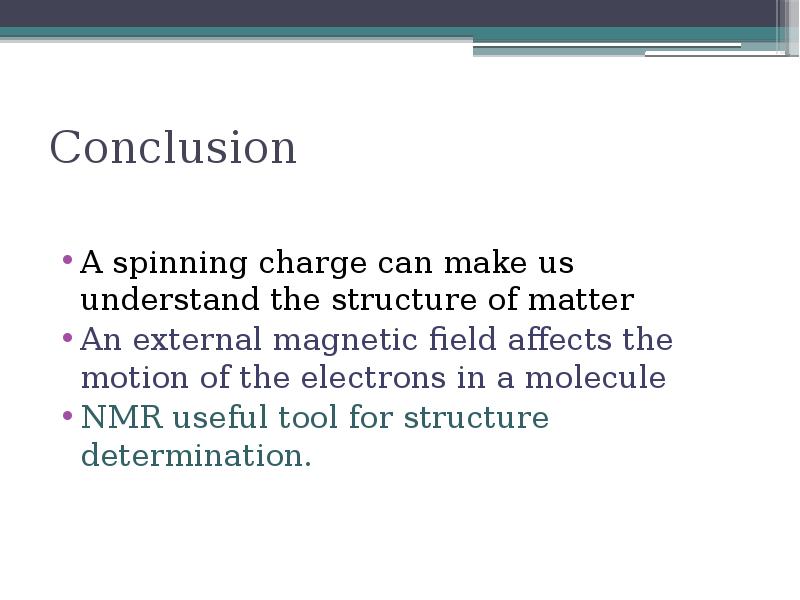
- 19. Conclusion A spinning charge can make us understand the structure of
- 20. THIS IS THE END. THX FOR ATTENTION.
- 21. Скачать презентацию




Слайды и текст этой презентации
Скачать презентацию на тему Nuclear magnetic resonance spectroscopy можно ниже:
Похожие презентации