Alkynes презентация
Содержание
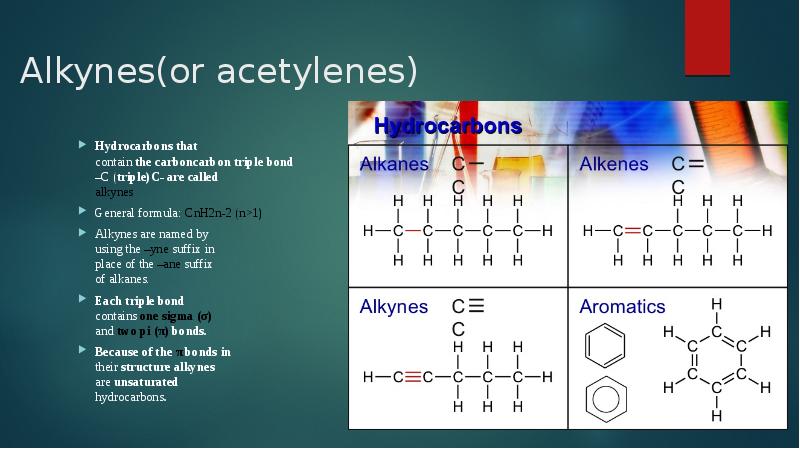
- 2. Alkynes(or acetylenes) Hydrocarbons that contain the carboncarbon triple bond –C (triple)C- are called alkynes General formula: CnH2n-2 (n>1)
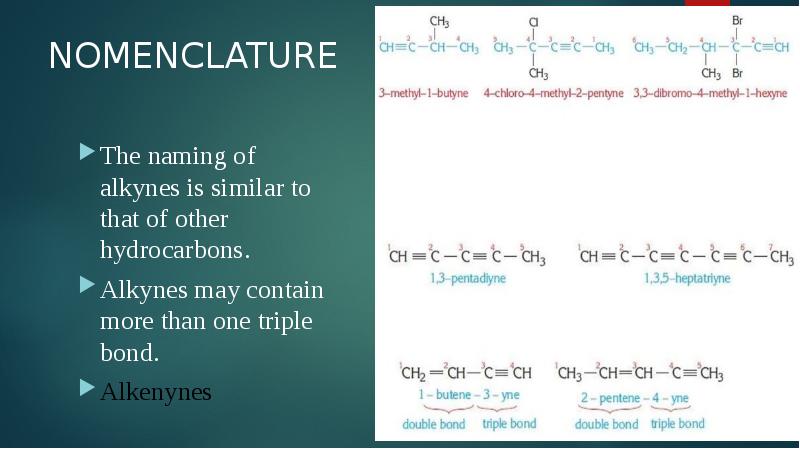
- 3. NOMENCLATURE The naming of alkynes is similar to that of other
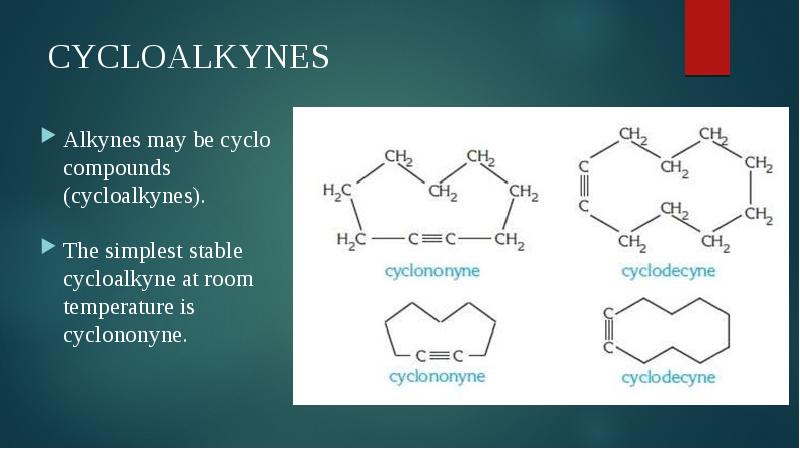
- 4. CYCLOALKYNES Alkynes may be cyclo compounds (cycloalkynes). The simplest
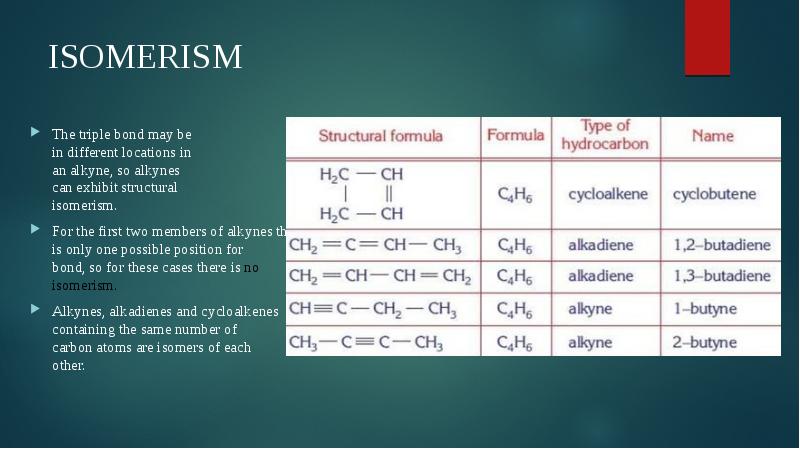
- 5. ISOMERISM The triple bond may be in different locations in an
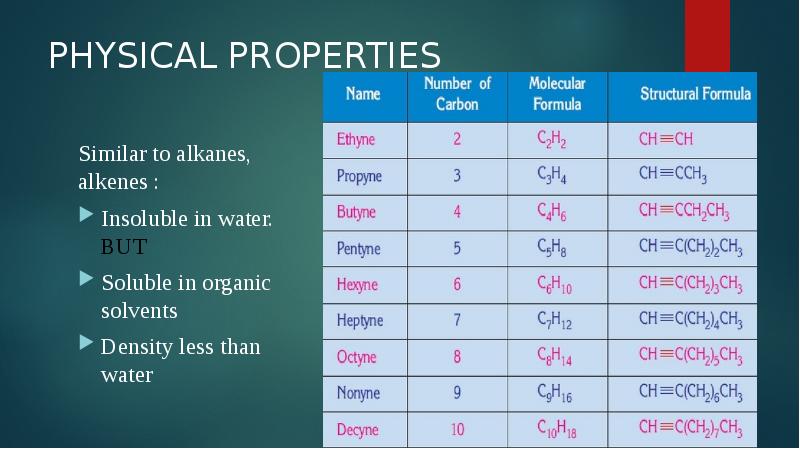
- 6. PHYSICAL PROPERTIES Similar to alkanes, alkenes : Insoluble in water. BUT Soluble in organic
- 7. CHEMICAL PROPERTIES Alkynes are unsaturated compounds and their chemical properties
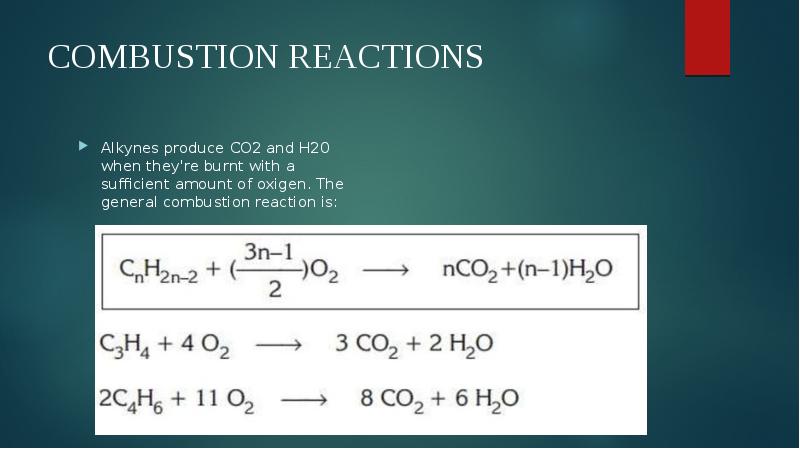
- 8. COMBUSTION REACTIONS Alkynes produce CO2 and H20 when they're burnt
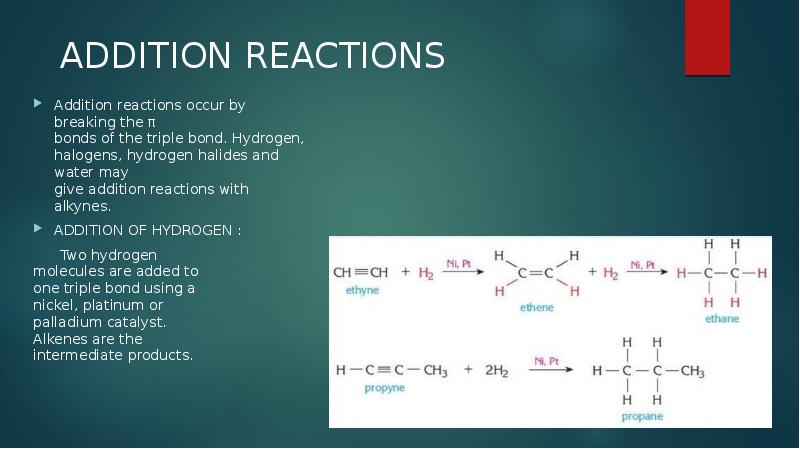
- 9. ADDITION REACTIONS Addition reactions occur by breaking the π bonds
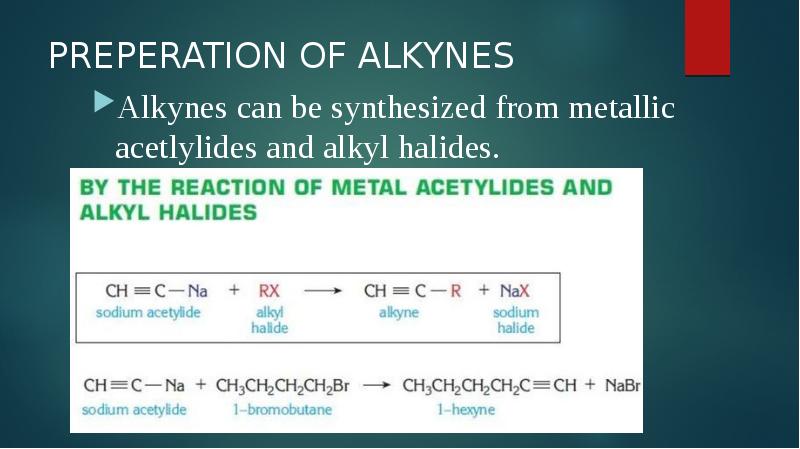
- 10. PREPERATION OF ALKYNES Alkynes can be synthesized from metallic acetlylides and alkyl halides.
- 11. ACETYLENE Acetylene, the first member of the alkyne series, is
- 12. ALKYNYL GROUP Alkynyl groups are formed from alkynes by removing
- 13. USES OF ALKYNES Histrionicotoxin toxic alkyne present in South American
- 14. Скачать презентацию





Слайды и текст этой презентации
Похожие презентации