Biochemical reaction kinetics презентация
Содержание
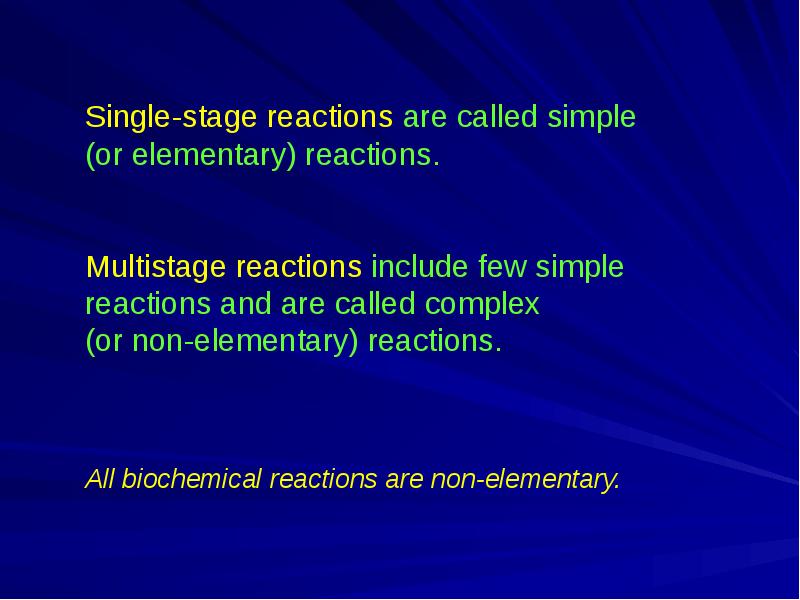
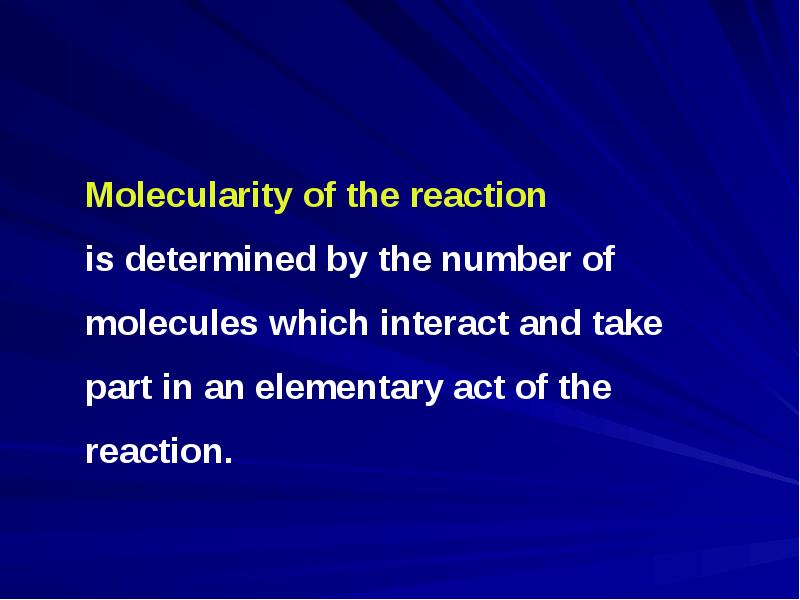
- 2. Chemical kinetics studies the rate and mechanism of chemical reactions Chemical
- 3. In homogeneous reactions all the reactants exist in the same phase
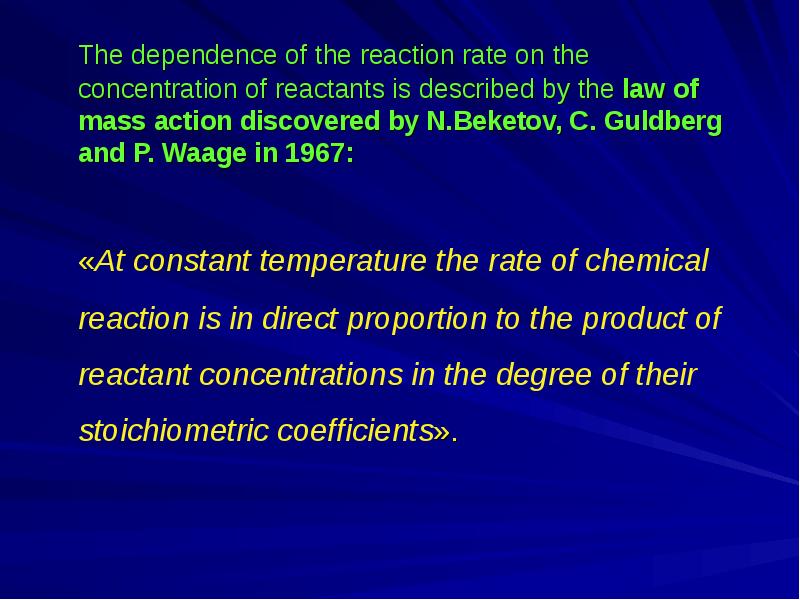
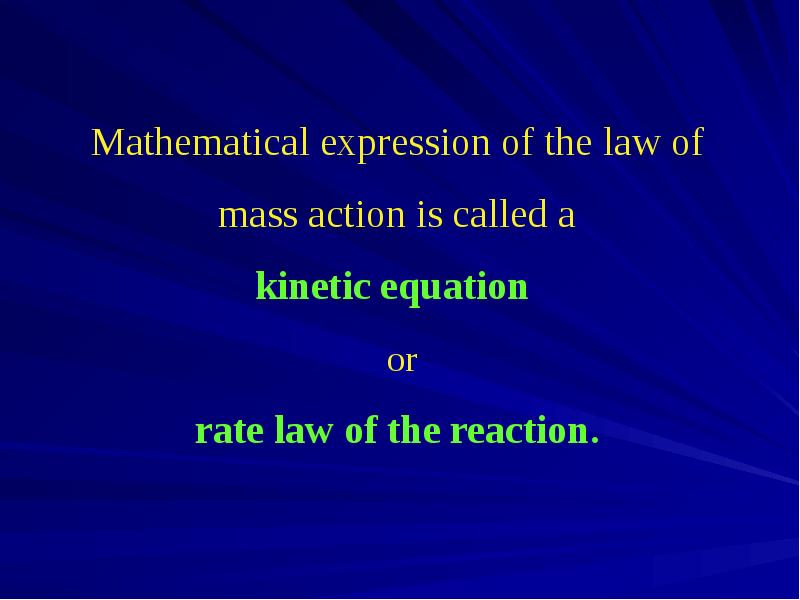
- 5. The dependence of the reaction rate on the concentration of reactants
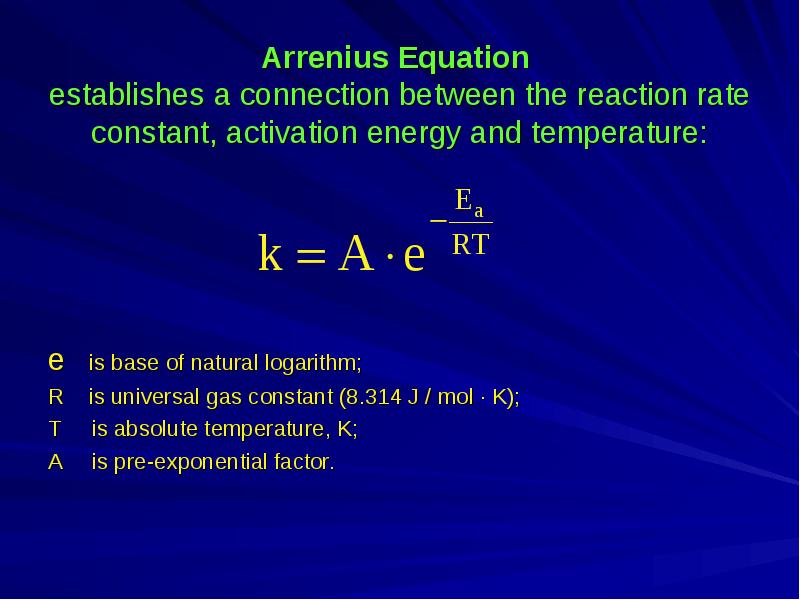
- 8. Arrenius Equation establishes a connection between the reaction rate constant, activation
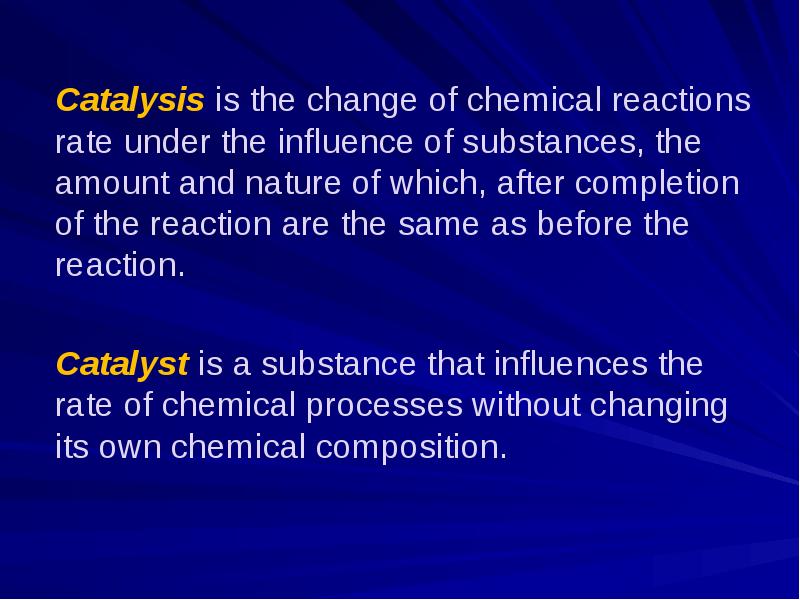
- 9. Catalysis is the change of chemical reactions rate under the influence
- 10. Enzymes are catalysts of the chemical reactions in the body.
- 11. Скачать презентацию




Слайды и текст этой презентации
Похожие презентации