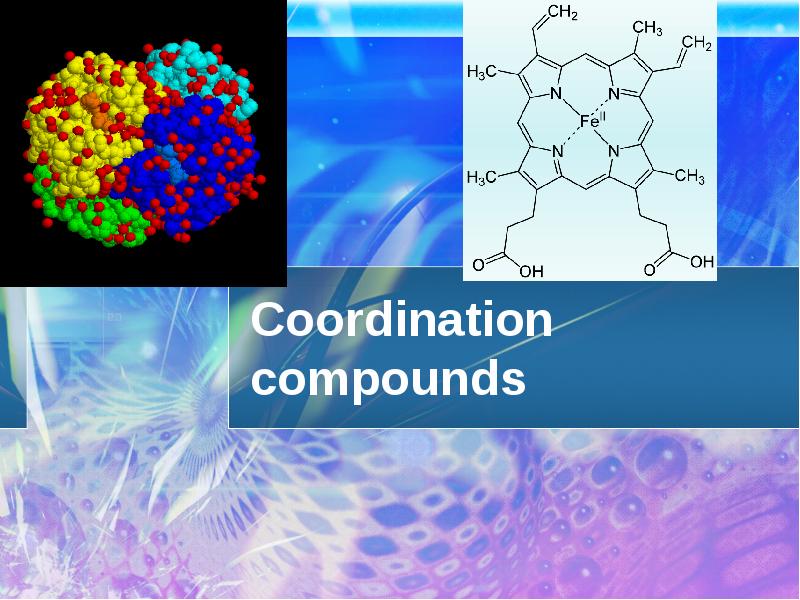
Coordination compounds презентация
Содержание
- 2. Processes of formation and destruction of complexes are used: in analytical
- 3. General information about coordination compounds According to their contents, chemicals are
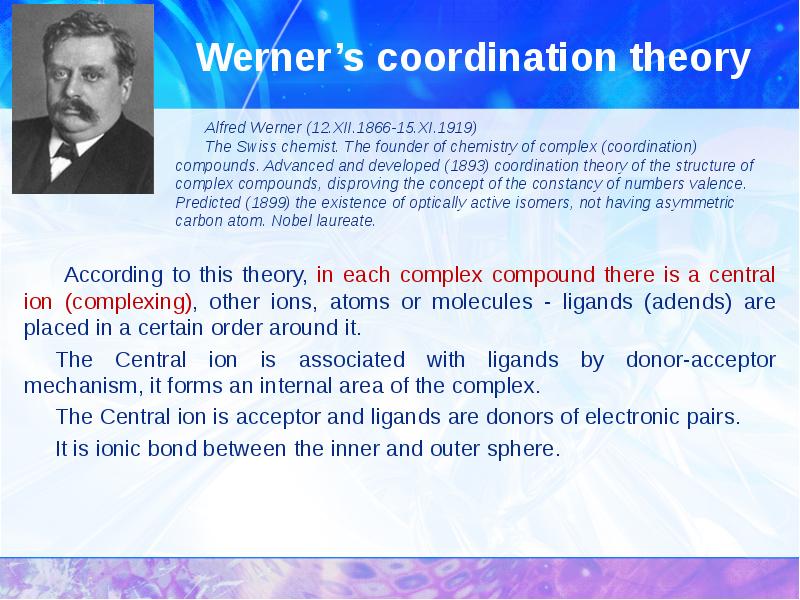
- 4. Werner’s coordination theory According to this theory, in each complex compound
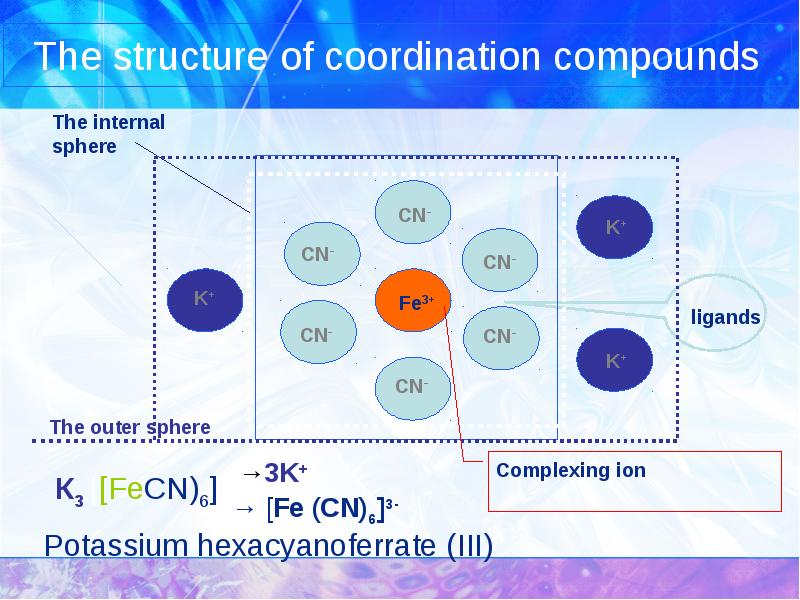
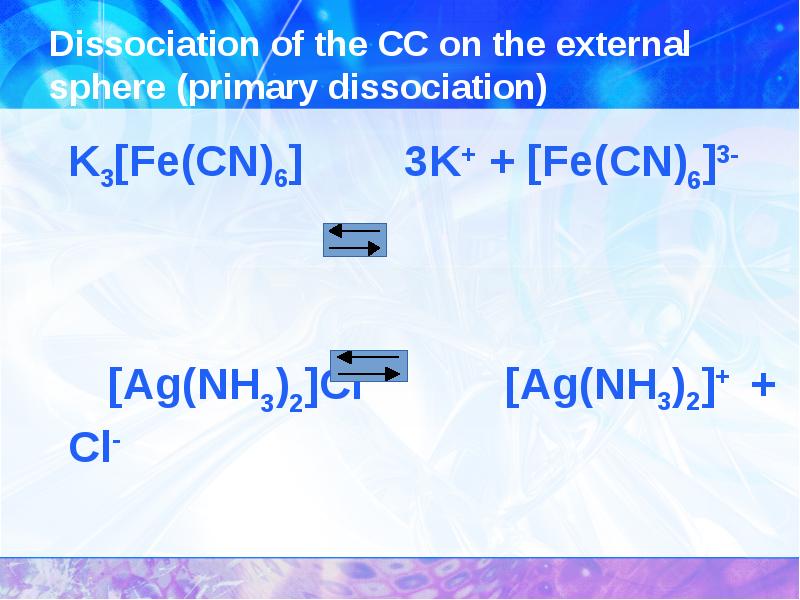
- 5. The structure of coordination compounds Potassium hexacyanoferrate (III)
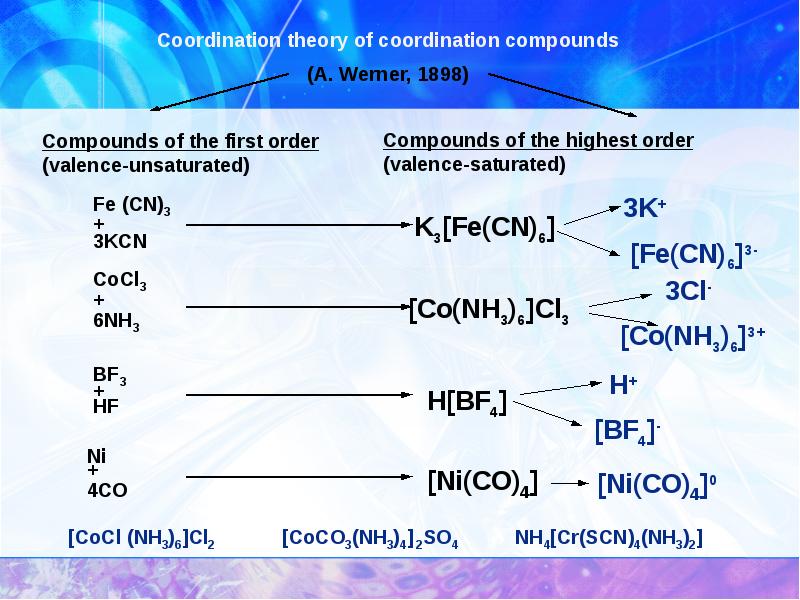
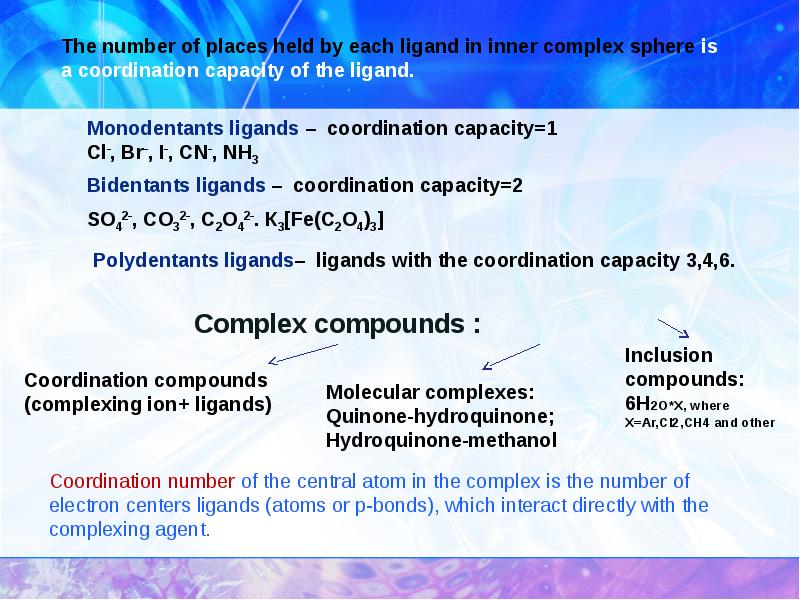
- 7. Complex compounds :
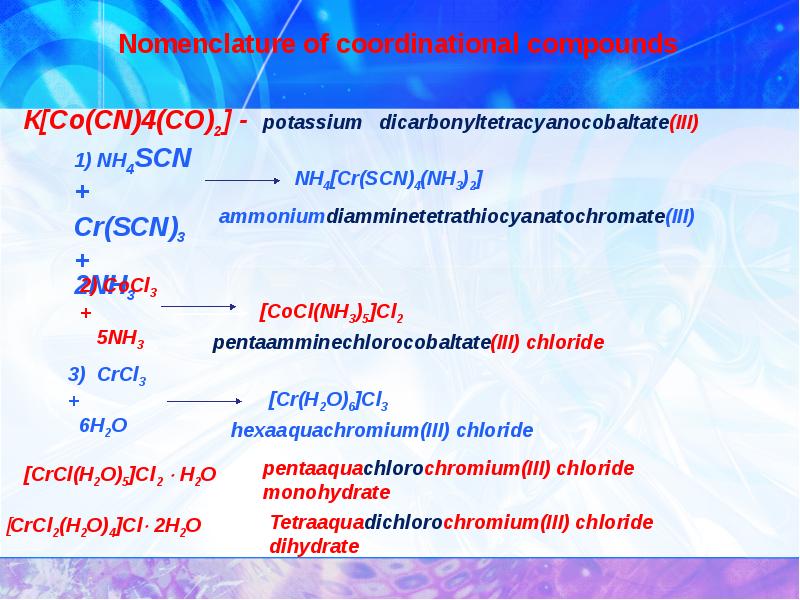
- 8. 2. Classification and nomenclature of coordination compounds
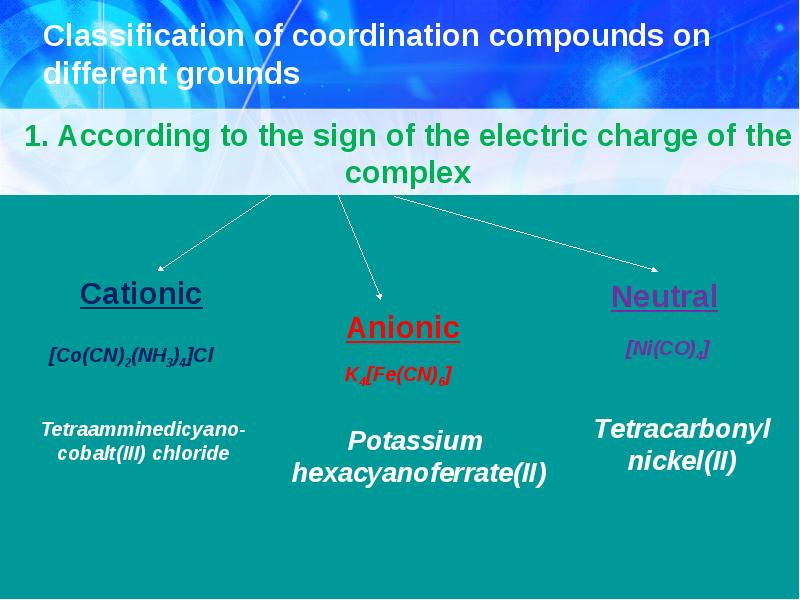
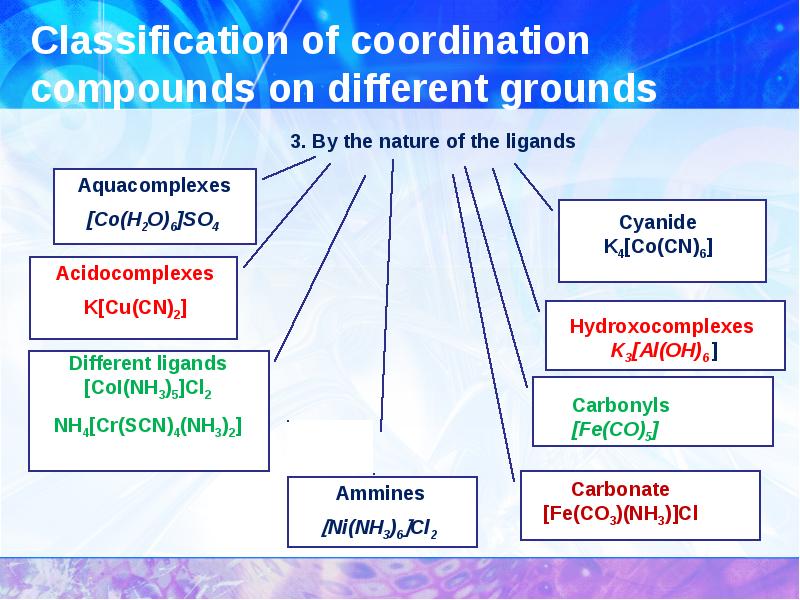
- 10. Classification of coordination compounds on different grounds
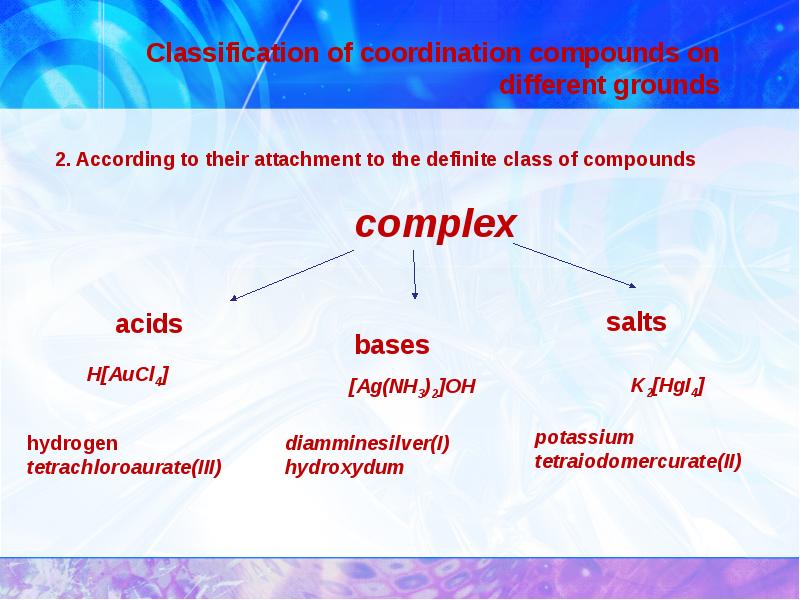
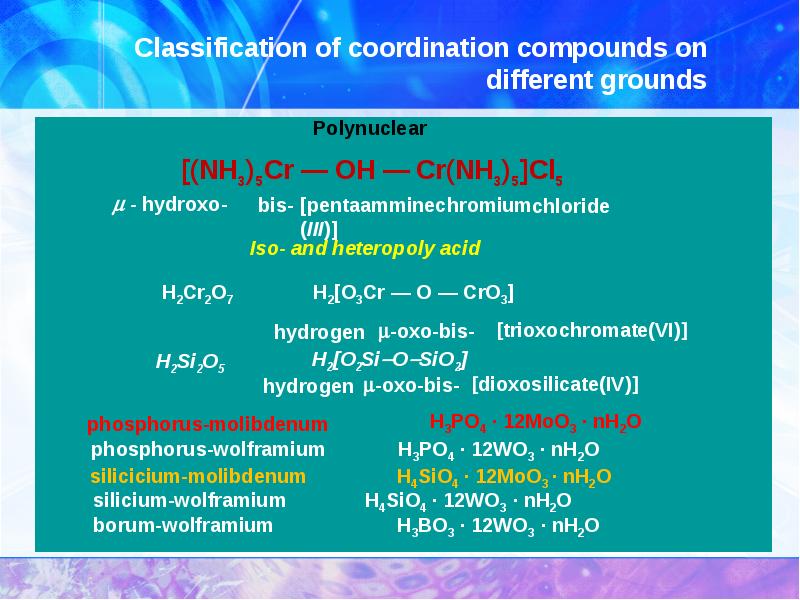
- 11. Classification of coordination compounds on different grounds
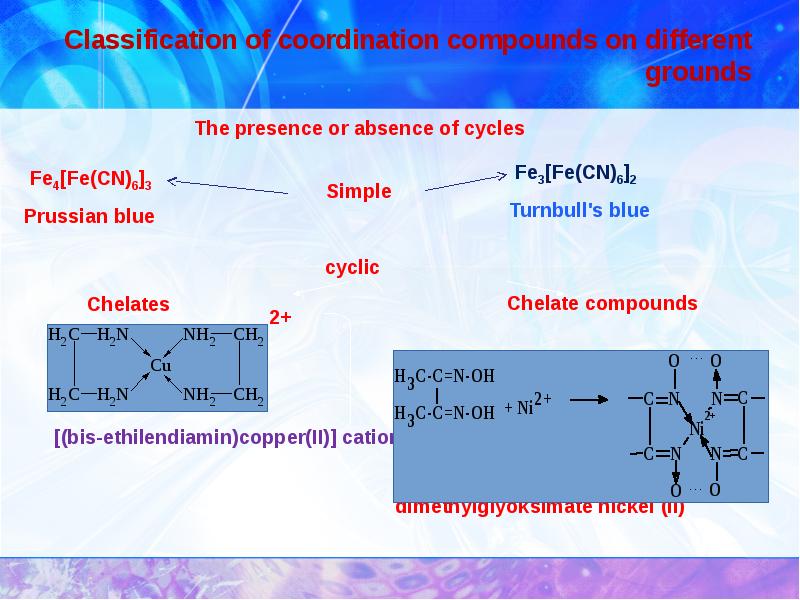
- 13. Classification of coordination compounds on different grounds
- 14. Classification of coordination compounds on different grounds
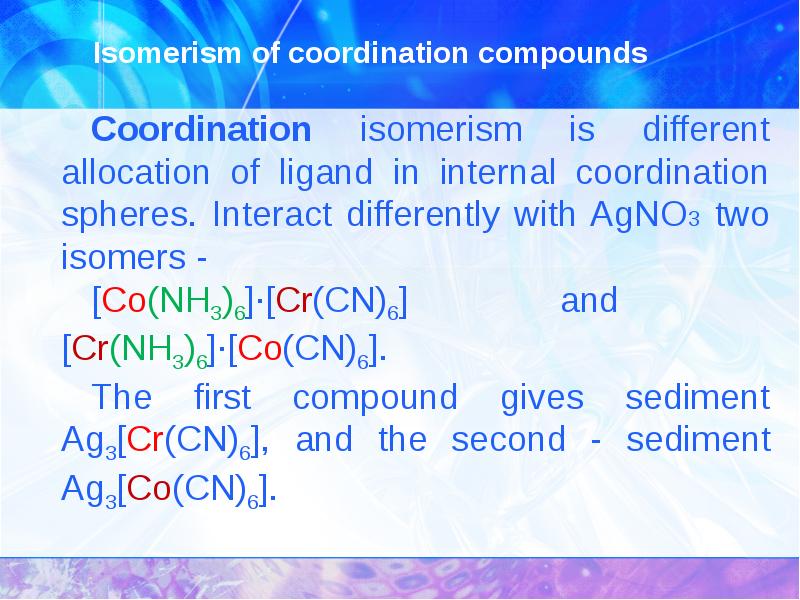
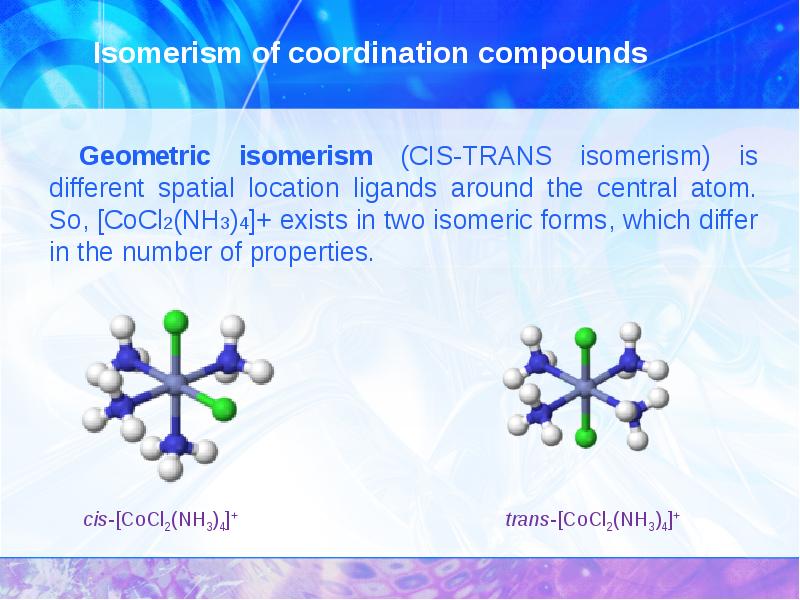
- 15. Isomerism of coordination compounds
- 16. Isomerism of coordination compounds
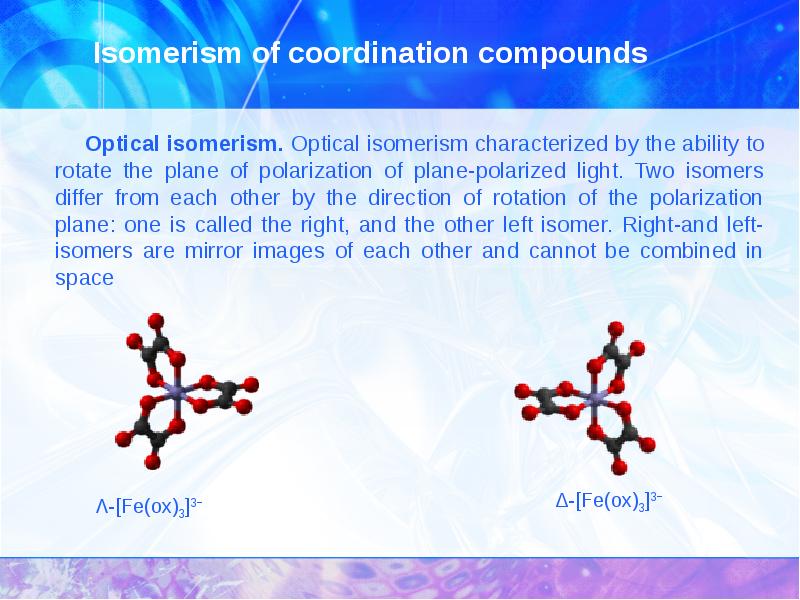
- 17. Isomerism of coordination compounds
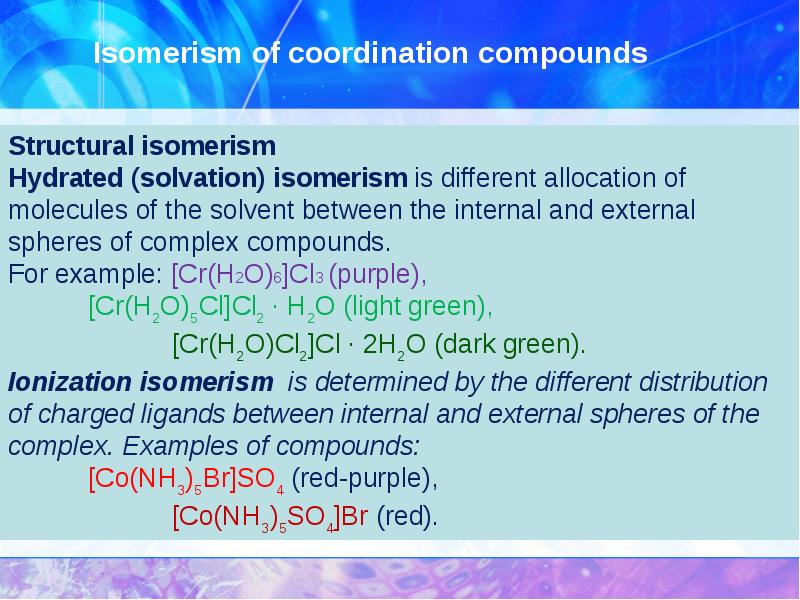
- 18. Isomerism of coordination compounds
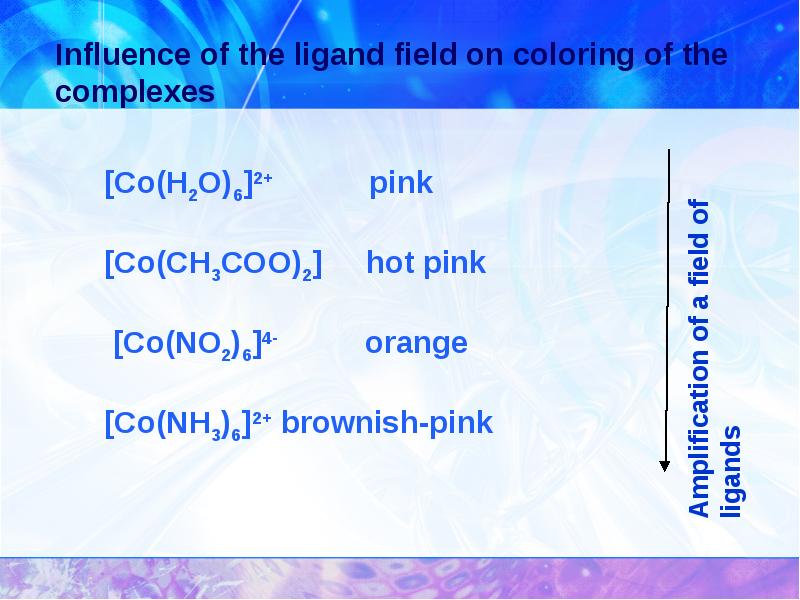
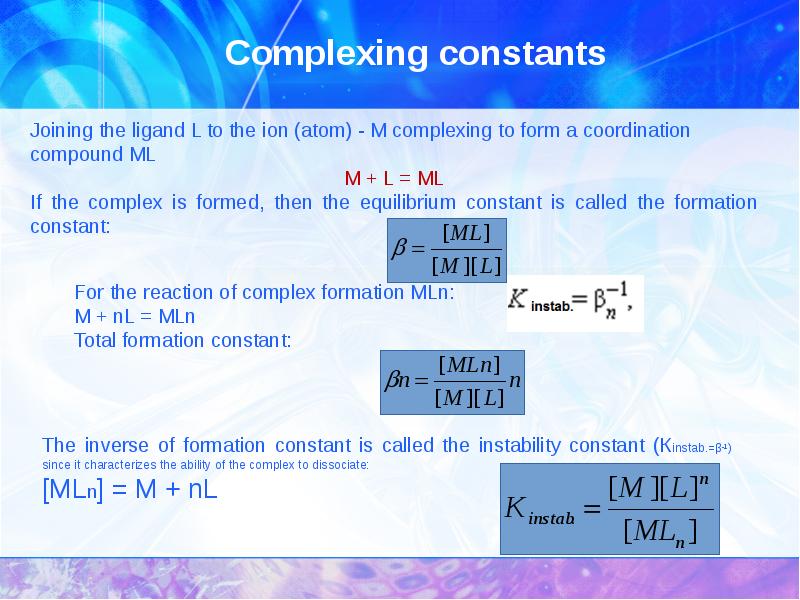
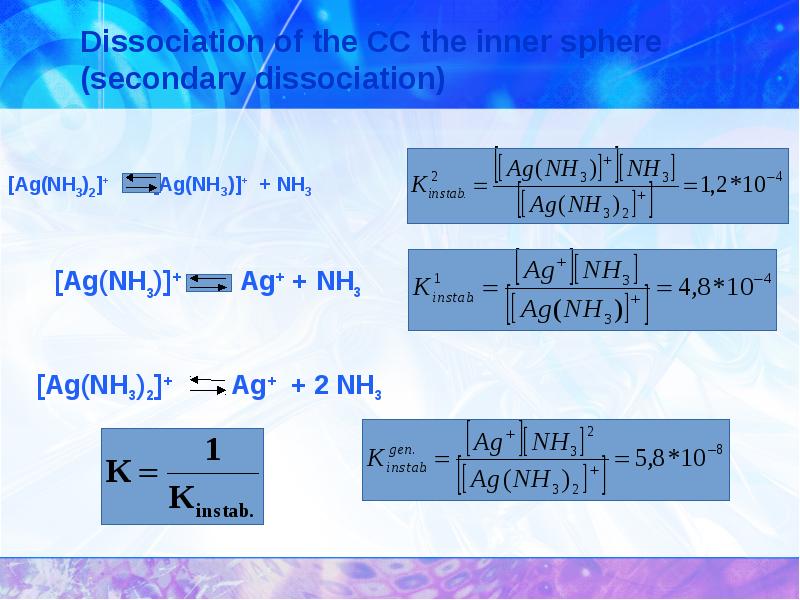
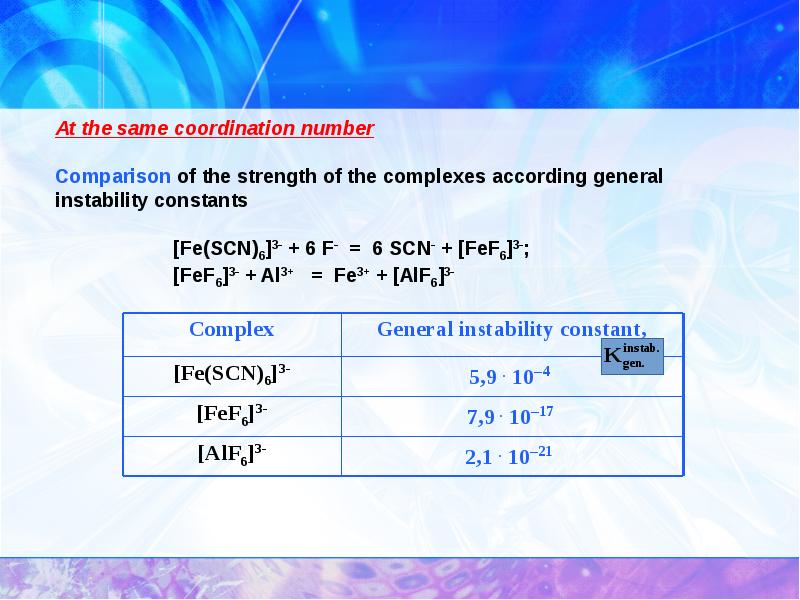
- 24. Complexing constants
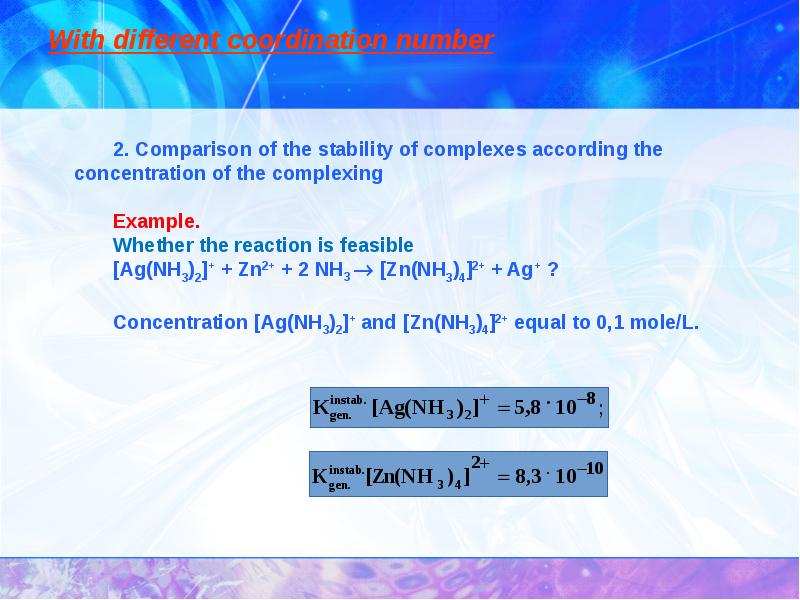
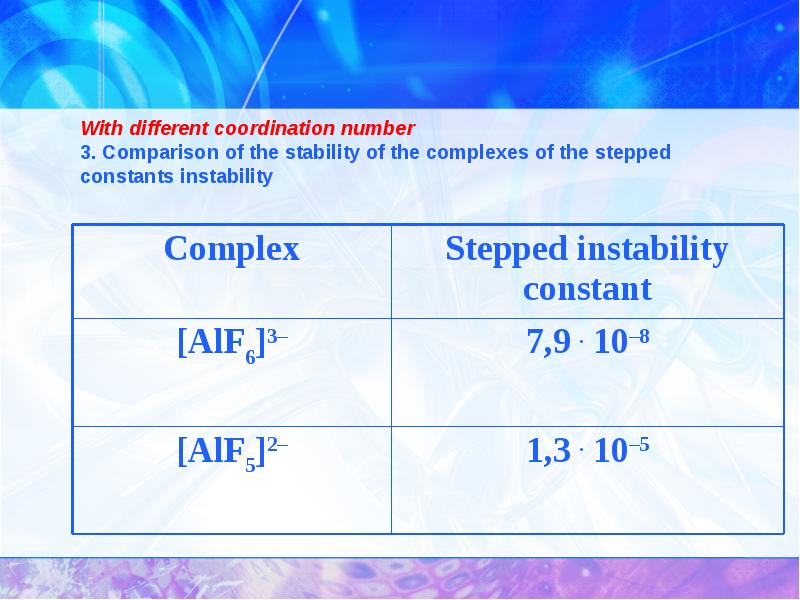
- 27. With different coordination number
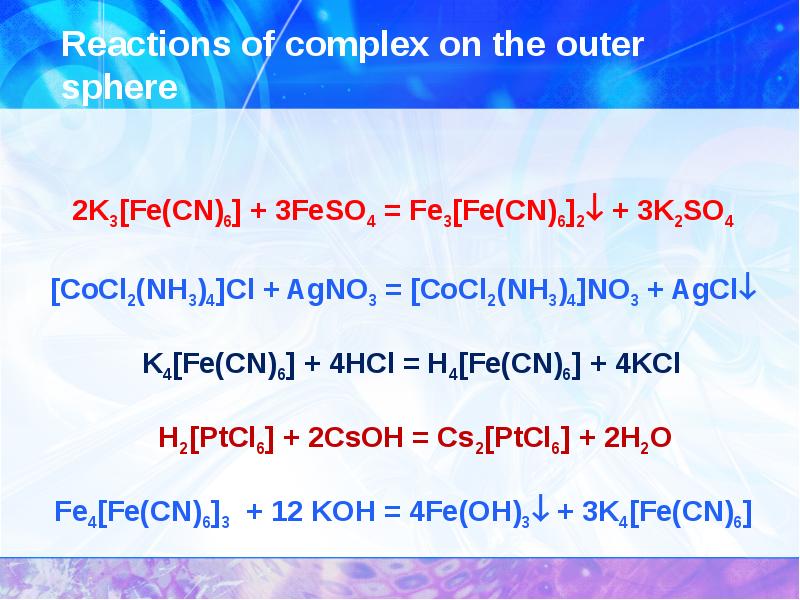
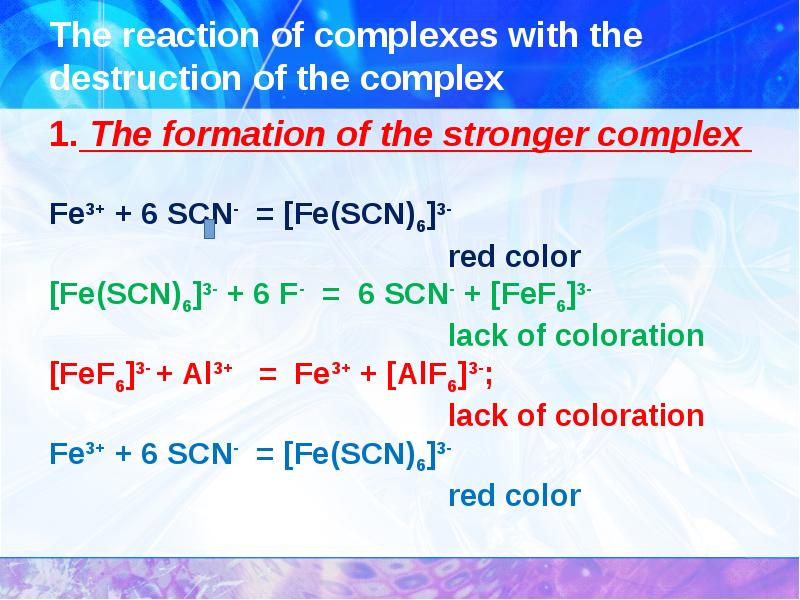
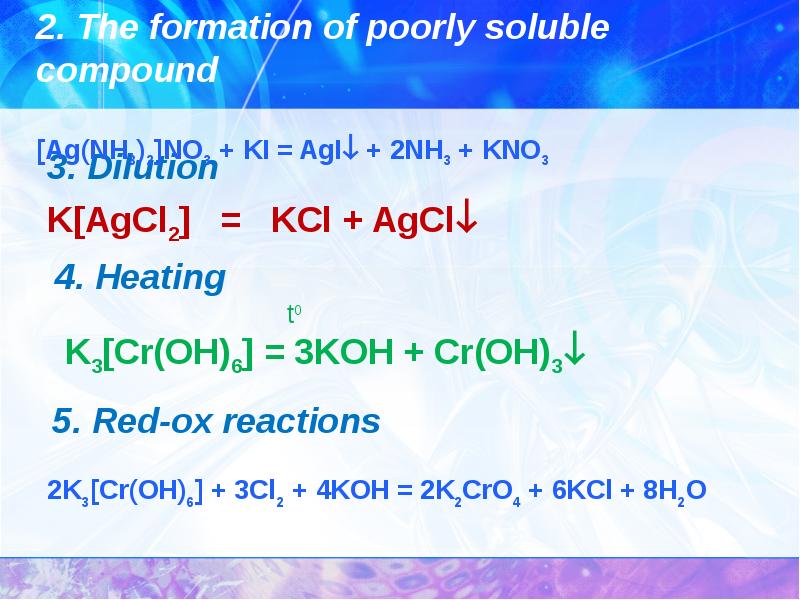
- 29. The reaction of complexes with the destruction of the complex
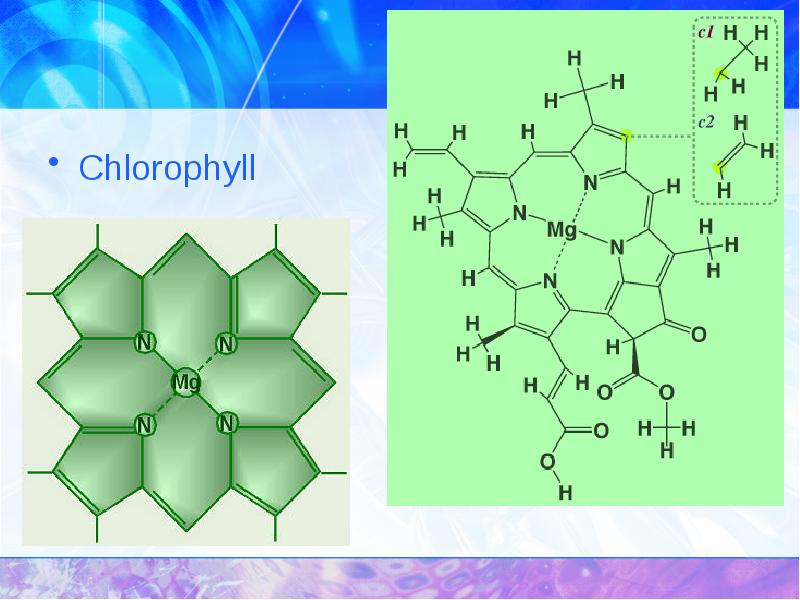
- 31. Chlorophyll Chlorophyll
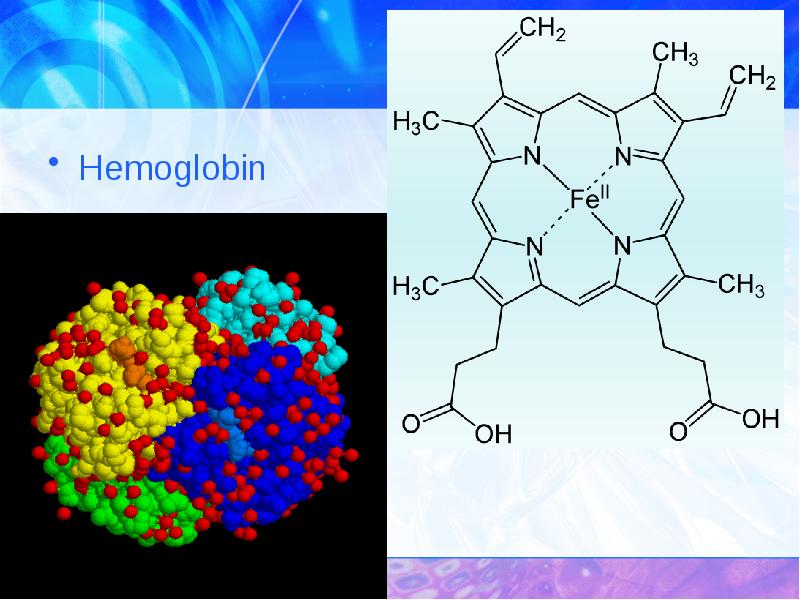
- 32. Hemoglobin Hemoglobin
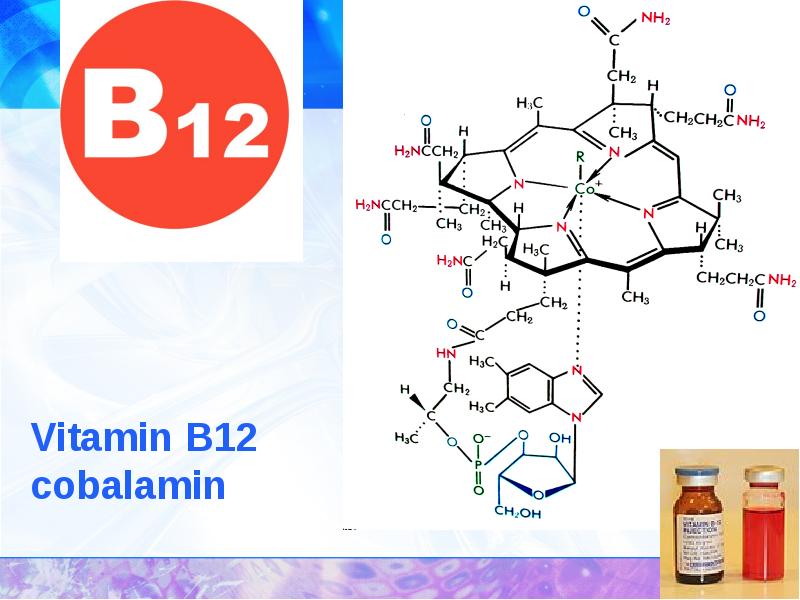
- 34. THE APPLICATION OF COMPLEXES IN MEDICINE
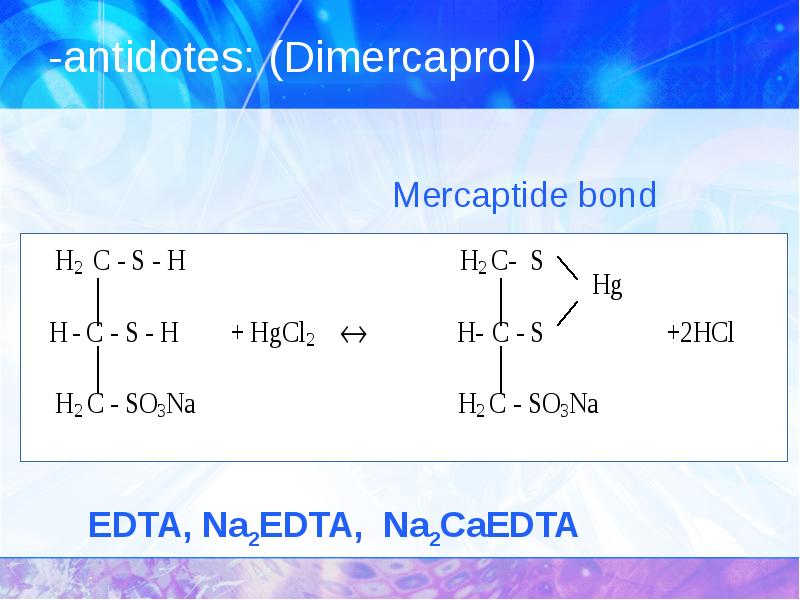
- 35. -antidotes: (Dimercaprol)
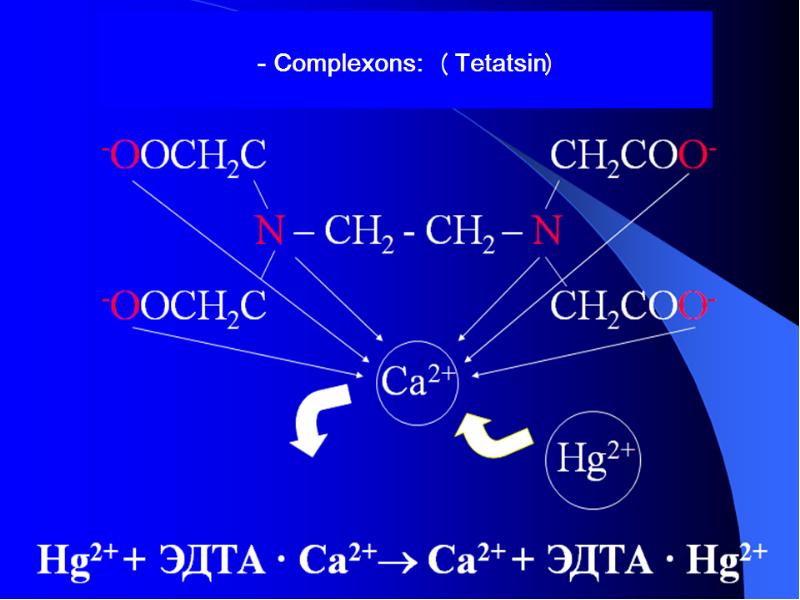
- 36. THE APPLICATION OF COMPLEXES IN MEDICINE For the lead removing using
- 38. -anticancer drug: dihlorodiamminplatinum cis-isomer (cis-platin) cis- [Pt(NH3)2Cl2] cis- [Pt(NH3)4Cl2]
- 39. The End
- 40. Скачать презентацию





![-anticancer drug: dihlorodiamminplatinum cis-isomer (cis-platin) cis- [Pt(NH3)2Cl2] -anticancer drug: dihlorodiamminplatinum cis-isomer (cis-platin) cis- [Pt(NH3)2Cl2]](/documents_3/3cb72d697aadd907145a913412d466de/img37.jpg)

Слайды и текст этой презентации
Похожие презентации