Sources of alkanes and cycloalkanes. Crude oil презентация
Содержание
- 4. Cracking Cracking converts high molecular weight hydrocarbons to more useful, low
- 6. Boiling Points of Alkanes governed by strength of intermolecular attractive forces
- 7. Induced dipole-Induced dipole attractive forces two nonpolar molecules center of positive
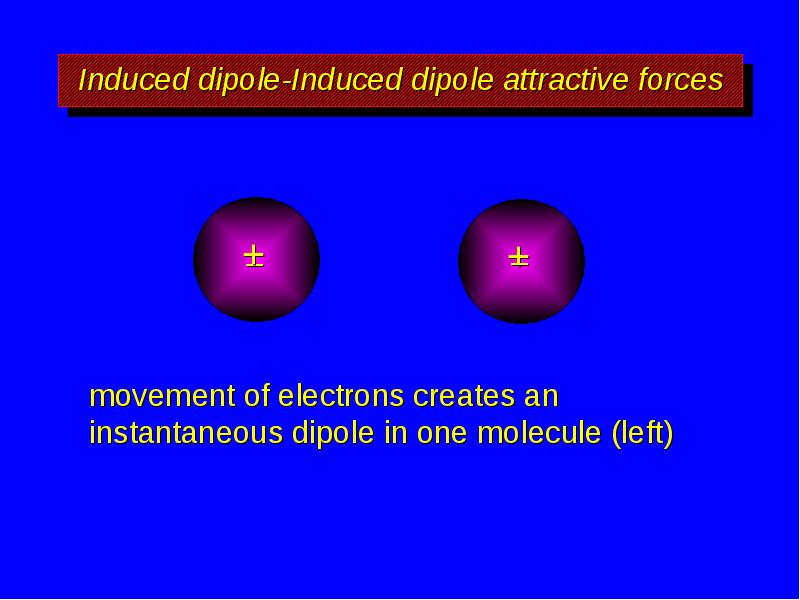
- 8. Induced dipole-Induced dipole attractive forces movement of electrons creates an instantaneous
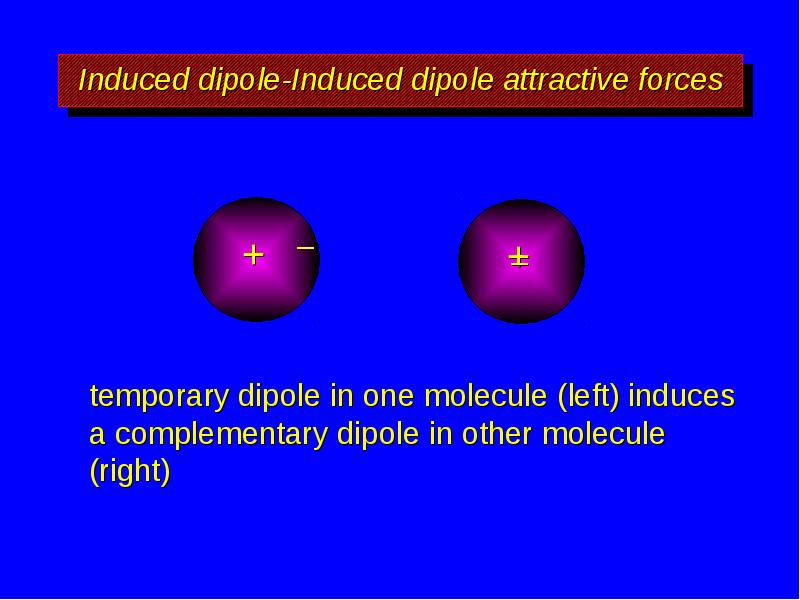
- 9. Induced dipole-Induced dipole attractive forces temporary dipole in one molecule (left)
- 10. Induced dipole-Induced dipole attractive forces temporary dipole in one molecule (left)
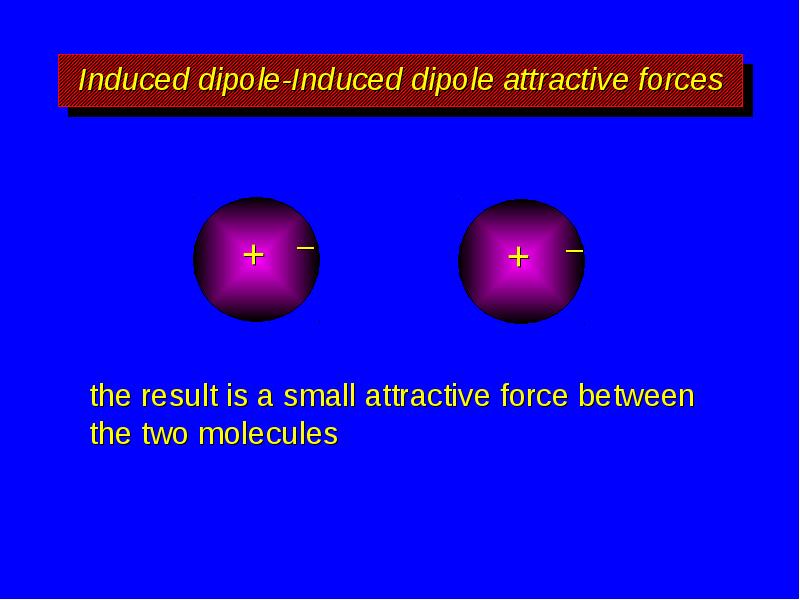
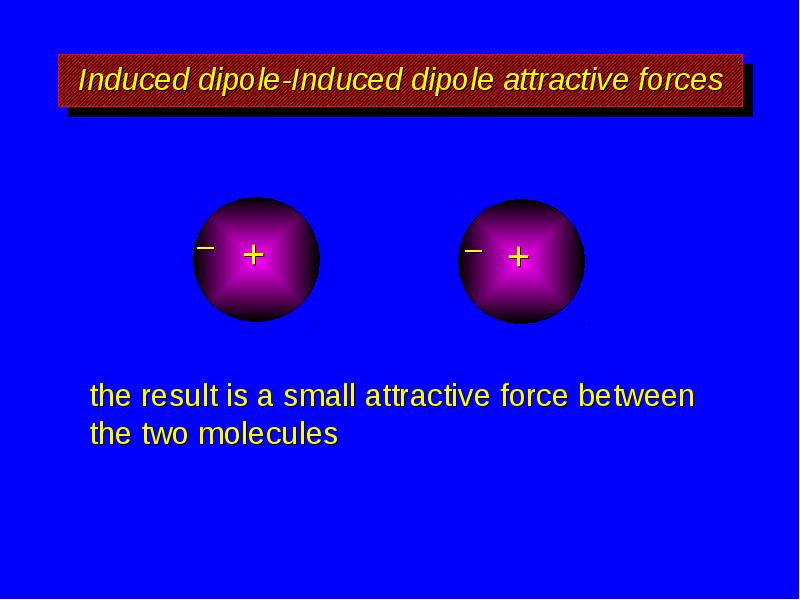
- 11. Induced dipole-Induced dipole attractive forces the result is a small attractive
- 12. Induced dipole-Induced dipole attractive forces the result is a small attractive
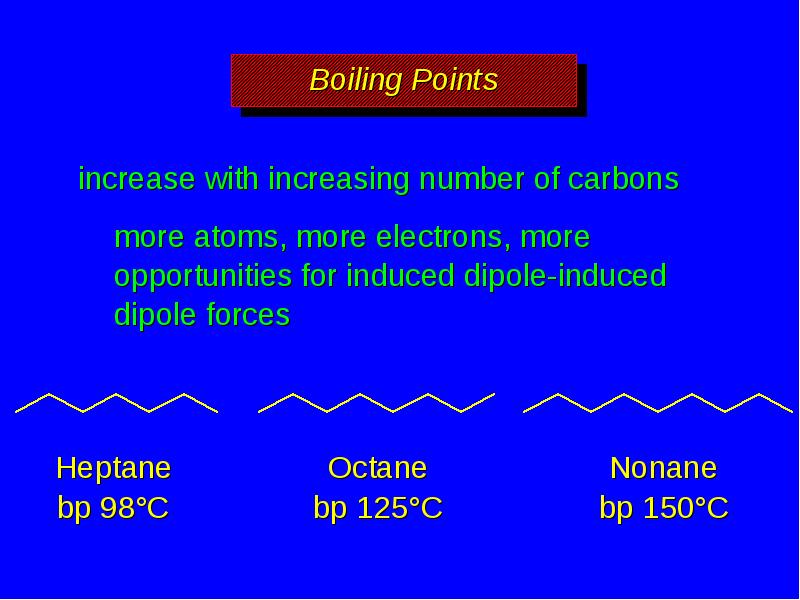
- 13. Boiling Points increase with increasing number of carbons more atoms, more
- 14. Boiling Points increase with increasing number of carbons more atoms, more
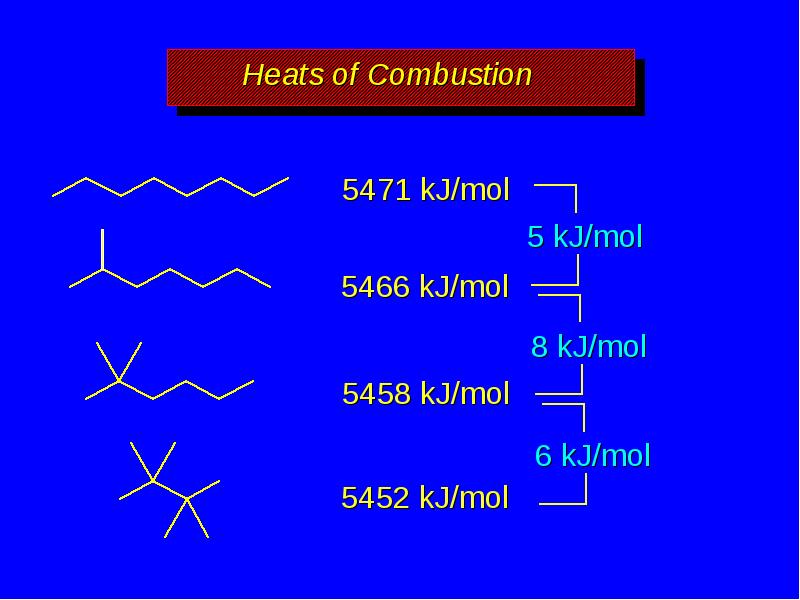
- 15. Boiling Points decrease with chain branching branched molecules are more compact
- 16. All alkanes burn in air to give carbon dioxide and water.
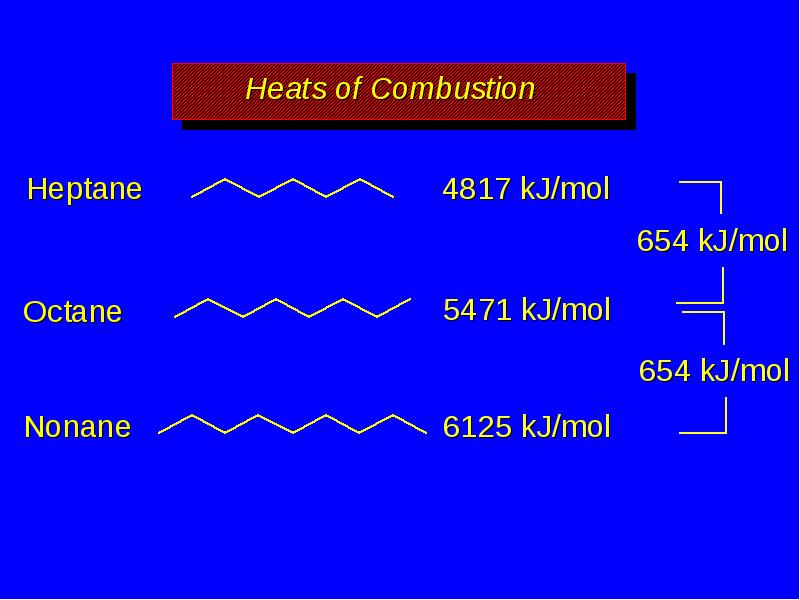
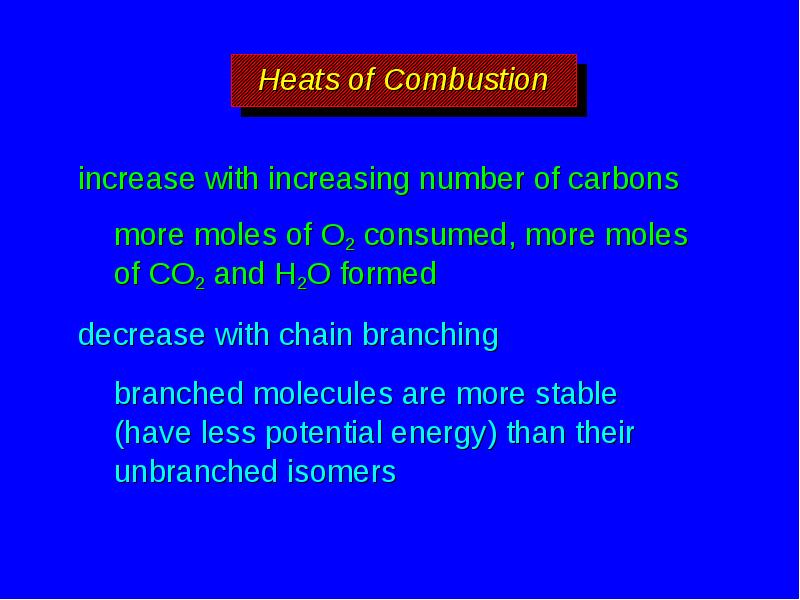
- 17. Heats of Combustion increase with increasing number of carbons more moles
- 19. Heats of Combustion increase with increasing number of carbons more moles
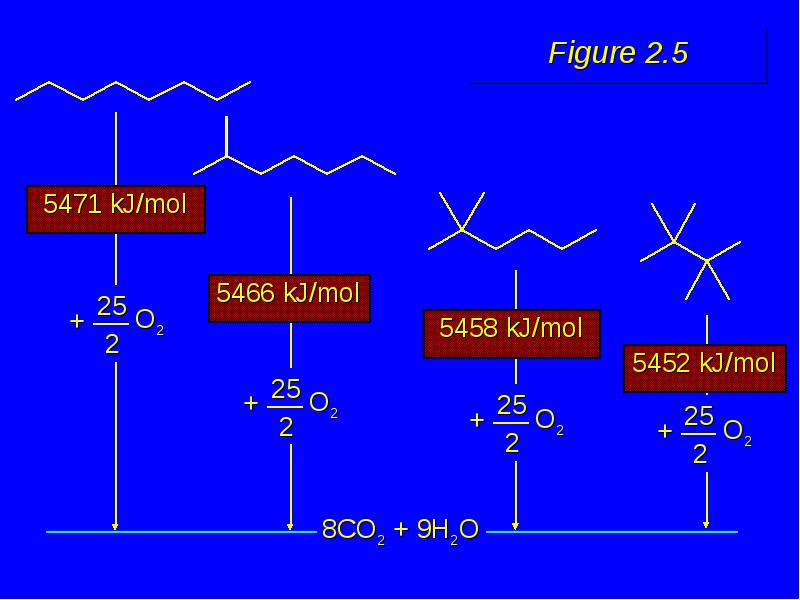
- 21. Important Point Isomers can differ in respect to their stability. Equivalent
- 22. Figure 2.5
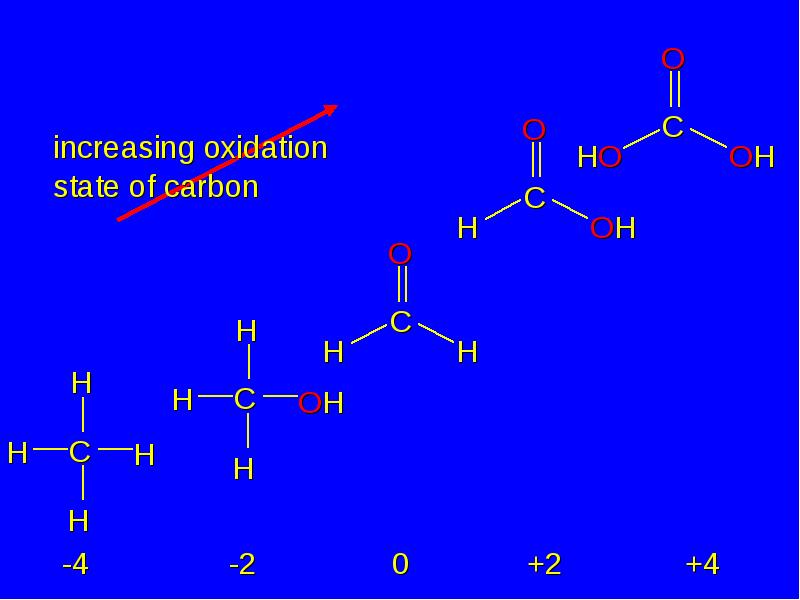
- 23. Oxidation of carbon corresponds to an increase in the number of
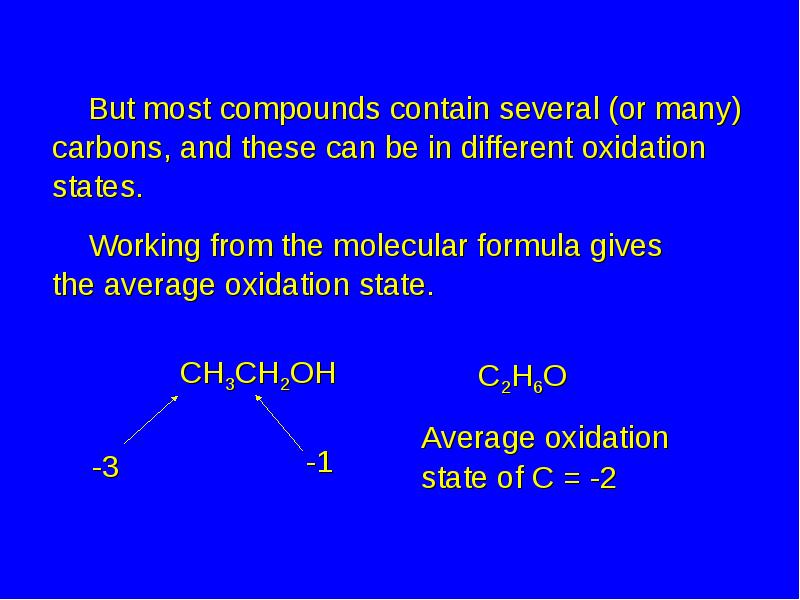
- 26. But most compounds contain several (or many) carbons, and these can
- 27. Fortunately, we rarely need to calculate the oxidation state of individual
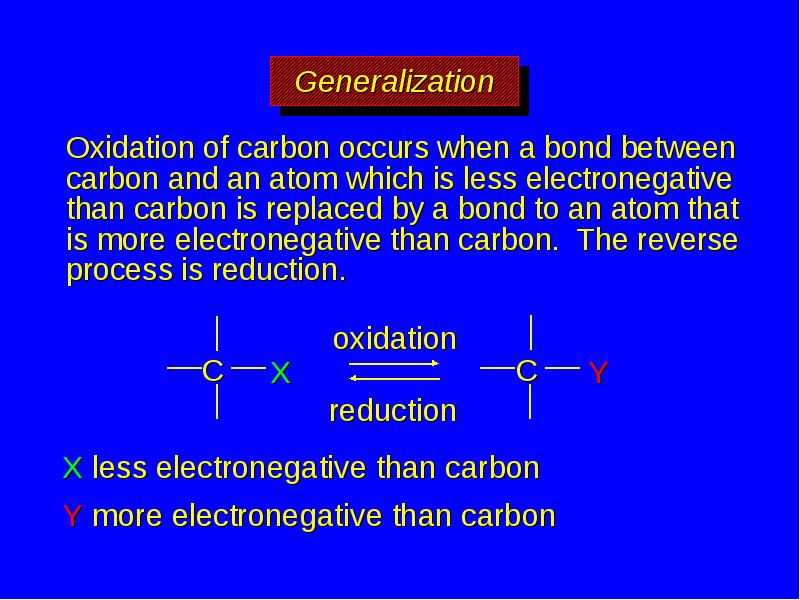
- 28. Oxidation of carbon occurs when a bond between carbon and an
- 30. Скачать презентацию

















Слайды и текст этой презентации
Скачать презентацию на тему Sources of alkanes and cycloalkanes. Crude oil можно ниже:
Похожие презентации